1234567890
prn8099 - Number 3, 1995

ALCOHOL - THE LEGAL DRUG OF ABUSE
by Abu Bakar Abdul Majeed PhD
Yesterday This Day's Madness did prepare;
To-morrow's Silence, Triumph, or Despair;
Drink! for you know not whence you came, nor why:
Drink! for you know not why you go, nor where.
Omar Khayyam
Alcohol is probably mankind's oldest drug. Surely, it is the oldest legal drug of abuse. Al-kohl is Arabic for finely ground antimony used as eye liner, and it came to mean any exotic essence. Over the years, physicians have prescribed it as a tranquilizer, sedative or hypnotic. This is because this drug is a central nervous system (CNS) depressant. Although alcohol is currently recognized as an official drug in the British and U.S. Pharmacopoeias, the various alcoholic beverages as such are no longer listed for medical use.
Alcohol is toxicologically important, not only as a poison in its own right, but because it is freely available and potentiates the CNS depressant effects of other psychotropic drugs. Today, alcoholism is considered as a disease. It is in fact a complex series of physiological, psychological and sociological disorders. The following excerpts from the National Geographic magazine aptly describe this chemical.
Raising a glass of alcohol is one of mankind's most distinctive rituals. For many, alcohol loosens the tongue and tightens the hands of friendship. Yet alcohol can also exert nearly satanic power: It ruins lives, destroys families, kills thousands on the highway. Each year more is known about this Jekyll and Hyde of the drug world. But alcohol is still a riddle that every culture attempts to solve in its own way.
Understandably, no country in this world is free from the alcohol menace. However, there is little data available on the extent of alcoholism or alcohol abuse in Malaysia. The problem is of greater concern in the Indian community who might have been introduced to toddy by the colonial plantation owners who wanted to keep their labourers under control and dependent.
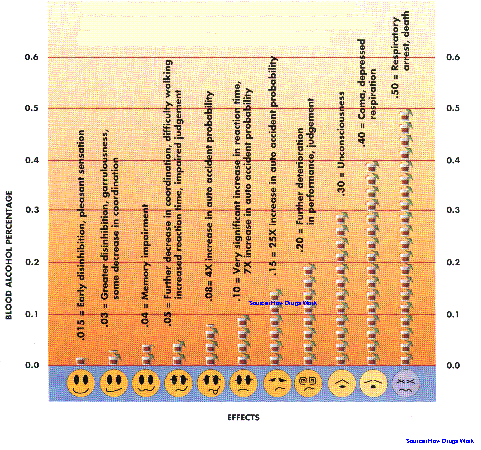
Today, alcoholism is a growing concern in the culture of social life in urban areas. For years, the most important social tragedy directly related to alcohol consumption is motor vehicle accidents. In a series of forensic post-mortems at Kuala Lumpur Hospital in 1991, it was reported that of the 59 road accident victims, 11 or 19% had blood alcohol levels greater than 100mg/dl. The concentration of alcohol in blood is usually given as % alcohol. This percentage refers to the number of grams (a gram is about 1/30 of an ounce) of alcohol in 100 mililioters (ml) (about 1/3 cup) of blood.Another study at the same hospital in the same year revealed that four out of every ten fatal car accidents were attributed to alcohol. Thirty percent of accident victims treated for head injuries were also under the influence of alcohol. In view of these grim statistics, the government has decided to nip the problem in its bud. Beginning July 1, 1995 the Motor Vehicle (Use of Breathlyser and Blood Urine Test) Rules will be enforced in the Klang valley. Under the law it would be an offence to drive, or attempt to drive if one's blood alcohol level is 80mg/dl (0.08%). At this alcohol blood level, a person will normally suffer from an impaired judgement of distances, impaired perception of red lights, braking and third lights, and an increased reaction time to emergencies. Drunk drivers also tend to drive faster and in a reckless manner, with little or no awareness of the peripheral areas. Situations which are normally easily avoidable, may lead to serious accidents when the drivers are under the influence of alcohol. However, motorists with blood alcohol level below 80mg/dl may not necessarily be able to drive safely, because even at a level of 20 mg/dl some sensitive drinkers may show symptoms of impaired concentration and mild motor incoordination. Factors like fatigue, illness, stress and drugs like the psychotropics, may potentiate the effects of alcohol.
The mental state of an intoxicated patient closely correlates with the blood alcohol level (see figure below). The blood level of alcohol is related to the volume of distribution and approximates whole body water, which is about 60-70% of body weight. The estimated oral dose that would cause the death of 50% of patients is that quantity producing a blood level of around 500mg/dl (0.50%). The "usual" 1-ounce drink of 80 proof (40%) alcohol in the 70kg individual will raise the blood alcohol by 25 mg/dl (0.025%). As in Malaysia, the definition for intoxication from alcohol is 80 mg/dl, thus the lethal/toxic ratio in nontolerant individuals is in the range of 6.25:1 (500 mg per cent: 80 mg percent). In fact, a mere three drinks are enough to make a person reach the statutory limit of intoxication.
The degree of acute alcohol intoxication depends on three main factors: the blood alcohol concentration, the rapidity of the blood alcohol rise, and the period during which the alcohol level is maintained. Alcohol is easily absorbed from the stomach into the bloodstream and rapidly distributed throughout total body water. From the blood it rapidly finds its way into the brain without restriction. After the blood alcohol level peaks, it declines at a relatively fixed rate as determined by metabolism, in which 95% is removed by oxidation and 5% removed via the lungs and kidneys. Most of the metabolism takes place in the liver under the influence of the enzyme alcohol dehydrogenase. The conversion of alcohol to acetaldehyde leads to the production of lactic acid. Lactic acid level is also enhanced due to alcohol-induced impairment of its uptake by the liver. This may initiate metabolic acidosis. Following an excessive drinking spree, alcoholic ketoacidosis is common especially in middle-aged women.
The most important single factor in the treatment of acute alcohol intoxication is the provision of respiratory and cardiovascular support. There appears to be no better substitute for supportive care. Gastric lavage, induction of emesis, and activated charcoal are not indicated because the drug is rapidly absorbed from the gastrointestinal tract and charcoal does not bind alcohol. Administration of stimulants, forced exercise, cold showers, or unusual remedies for curtailing alcoholic intoxication should be avoided.
When alcohol consumption is discontinued abruptly, a characteristic syndrome of motor agitation, anxiety, insomnia, and reduction of seizure threshold occurs. The later, severe withdrawal syndrome occurring usually 48-72 hours after cessation of drinking is known as delirium tremens. This is characterised as visual hallucinations, confusion, disorientation and autonomic overactivity. These symptoms can be treated by the benzodiazepines. Nutritional supplements are also essential, especially potassium, magnesium and phosphate.
Scientists have made great strides toward understanding how alcohol acts on the brain. This knowledge has launched a burgeoning era of research efforts to develop medications that impede the progress of alcoholism and lessen the risk of relapse to the disease. Some of the research strategies adopted in developing drugs to combat this menace include altering drinking behaviour to control craving and prevent relapse, inducing sobriety in intoxicated patients, treating alcohol-induced cognitive impairment, managing acute alcohol withdrawal syndrome and treating coexisting psychiatric disorders. It is hoped that the new drugs to be developed from these studies can improve long-term treatment outcome when coupled with traditional behavioural therapies.
Dr Abu Bakar is an Associate of PRN specialising in Neurotoxicology. He is the CECT Editor.
PRN CONSULT 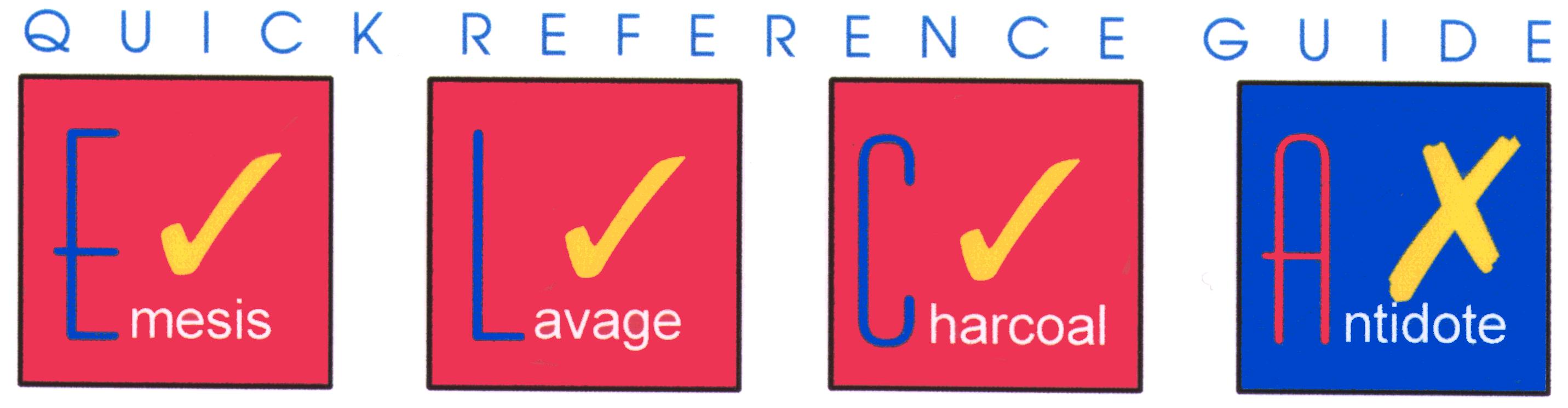
Review Of Salicylate Poisoning
Abas Hj Hussin, PhD
Salicylates are present in the barks of trees belonging to the willow, Salix alba and certain other families. In fact, the word 'salicylate' is derived from the botanical name for the willow family, Salicaceae. Salicylic acid was first isolated from willow bark in 1838, but not until 1899 that acetylsalicylic acid was first synthesized by Hoffman of the Bayer Company and was introduced into medicine under the name, Aspirin. Aspirin tablets are available in enteric-coated, buffered preparations or effervescent tablets. Aspirin is one of the cheapest, most easily available and most widely used drugs in the world.
Salicylates are found in a variety of prescription and over-the-counter analgesics, cold preparations, cold sore preparation and topical keratolytic products (see Table 1).
| Table 1 Some common products containing salicylate |
|---|
Source : DIMS Volume 24 Number 1, 1995
They are frequently combined with other drugs such as phenacetin, codeine, caffeine, and acetaminophen in different proprietary preparations. They are also available in a variety of other preparations. For example methyl salicylate (oil of Winter Green) is still used externally, as a counter-irritants in embrocations and liniments (commonly known as LMS) which can be readily obtained from retail pharmacy outlets, government or private clinics. It is popularly used in traditional medicine preparations such as in the massage oil (or 'Minyak Urut') and medicated oil.
Incidence of salicylate poisoning has been on the decline, chiefly because its use as analgesic has been surpassed by paracetamol. However, 27 cases of salicylate poisoning at the Hospital Besar Kuala Lumpur was reported in 1987. Throughout 1979-1988, the Department of Chemistry, Malaysia reported a total of 154 methyl salicylate, 103 salicylic acid, 8 salicylamide and 7 acetylsalicylic human poisoning cases. There are four groups who are considered at risk for salicylate poisoning : accidental acute paediatric ingestions; a child is overzealously treated with salicylates by an anxious parent during the first few days of an illness, resulting in chronic toxicity; single large ingestion taken as an attempted suicide; and elderly patients who suffer chronic toxicity following a gradual alteration in the patient's metabolic processes or to drug interaction that enhance the toxicity of salicylate.
What are the clinical manifestations of salicylate toxicity?
The two most important factors in the type of clinical syndrome produced by salicylates are the age of the patient and the dose taken. Children under the age of 12 years rarely develop respiratory alkalosis; the picture is usually that of a metabolic acidosis.
In the early stages of poisoning the most notable features are vomiting and epigastric pain. Nausea and vomiting may result from direct gastrointestinal irritation. It cannot be emphasized too strongly that coma is a very late feature of this type of poisoning and that a lethal dose of salicylate may have been taken but the patient remains fully conscious. Coma is rare and is generally only seen in massive ingestions (serum salicylate levels greater than 100 mg/100 ml) or in mixed (together with other drugs) overdoses.
As serum salicylate levels increase, tinnitus followed by diminished auditory acuity occurs, both of which are due to direct ototoxicity. The victims sweat profusely and are flushed, with warm extremities and bounding pulses. Uncoupling of oxidative phosphorylation increases heat production, the basal metabolic rate, oxygen consumption, and cardiac output. Any elevation in temperature must be regarded as an indication of severe toxicity and merits close monitoring of such a patient. The increase in metabolic demands stimulate peripheral utilization of glucose, with resultant hypoglycemia. There may be a transient period of hyperglycaemia that results from an impaired ability of tissues to utilize glucose. Serum hypoglycaemia as well as CNS hypoglycaemia is common during chronic intoxication or late in the course of an acute ingestion. There is usually obvious hyperventilation, with both the rate and depth of respiration being increased. Dehydration occurs quickly as does hypokalaemia. The latter, which may produce a fatal cardiac arrythmia, seems to be produced by a combination of alkalosis, vomiting and an increased output of cortisol from the adrenal gland.
Central nervous system toxicity tends to develop rather late, as the accumulating metabolic acidosis allows salicylate to penetrate the brain more easily. Initially tremor appears followed by hallucinations, delirium and drowsiness. One dangerous complication is that of pulmonary oedema and this can occur even in the face of hypovolaemia. In this case it may be due to damage to the pulmonary vascular capillaries resulting in abnormally increased permeability to plasma. Bleeding has been described, caused probably by a combined effect on platelet aggregation and liver synthesis of coagulation factors, such as prothrombin. Finally acute oliguric renal failure and inappropriate secretion of antidiuretic hormone may complicate the clinical picture. Hepatic toxic reactions are reported, but none has resulted in permanent residual effects. Death is due to central nervous system failure and cardiovascular collapse. Morbidity and mortality rates are much higher in chronic intoxication than after acute overdose. Patients with chronic ingestions may have a mortality rate as high as 25% (versus an acute ingestion mortality rate of < 1%). Cerebral and pulmunory oedema are more common than with acute intoxication, and severe poisoning occurs at lower salicylate levels.
How do you diagnose and assess the severity of salicylate poisoning?
The diagnosis of salicylate poisoning is usually straightforward as a result of the fact that respiration is stimulated, which distinguishes the overdose from that due to barbiturates or opiates. The type of breathing is very similar to that seen in diabetic ketoacidosis. Diagnosis is usually not difficult if there is a history of acute ingestion, accompanied by typical signs and symptoms. In the absence of a history of overdose, diagnosis is suggested by the characteristic arterial blood gases, which reveal a mixed respiratory alkalemia and metabolic acidosis.
Altered consciousness is the most important indicator of severe intoxication. This feature is rarely encountered in older children and adults, but they commonly complain of tinnitus and deafness and showing sweating (diaphoresis) and hyperventilation (see Table 2).
Table 2 Symptoms and toxicity levels of salicylate poisoning
| Symptoms | Classification of Toxicity |
|---|---|
| Asymptomatic Mild intoxication Moderate intoxication Severe intoxication |
No objective findings; subjective complaints Hyperpnea (mild), tinnitus, vomiting, with or without lethargy Hyperpnea, severe neurologic, acid-base, GI and coagulation abnormalities Hyperpnea: deep coma, convulsions, cardiovascular collapse |
From Done AK: Salicylate intoxication: Significance of measurements of salicylate in blood in cases of acute ingestion. Paediatrics 26:800, 1960.
The acid base status of the patient should also be established together with the blood urea and electrolytes. Blood sugar and ketones should be determined if there is any question of diabetes mellitus. Other useful laboratory studies include anion gap calculation, creatinine, liver function tests, prothrombin time and chest x-ray. Abdominal x-ray may reveal radiopaque enteric coated or sustained release tablets.
For acute ingestion, salicylate levels are plotted on the Done nomogram (Figure 1) to determine expected toxicity. A single ingestion of less than 150 mg/kg generally results in negligible clinical toxicity. An ingestion of 150-300 mg/kg may result in mild to moderate toxicity. An ingestion of more than 300 mg/kg results in prolonged and severe effects, including severe hyperpnea, and prominent neurologic disturbances. Ingestions of more than 500 mg/kg are considered potentially lethal. Toxicity also developed in those with chronic administration of more than 100 mg/kg/24hr for 2 days or more. Single salicylate level determination is not sufficient because of the possibility of delayed absorption from sustained-release tablets.
Methyl salicylate is much more toxic when taken by mouth. In young infants, 2 to 5 ml has been fatal and in adults 5 to 30 ml. This is presumably because of the very rapid absorption of salicylate in this form. However, the Done nomogram is not useful for patients with chronic ingestions, as salicylate levels do not correlate well with the patient's clinical status. In addition, the nomogram is not useful prior 6 hours after ingestion.
A rapid qualitative test for the presence of salicylates may be done by adding several drops of 10% ferric chloride to 1 ml of boiled urine. The urine will give a purple colour with ferric chloride or Phenistix (Ames). This dipstick turns brown with presence of salicylates as well as phenothiazines. The addition of 1 drop of 20 N sulphuric acid to the strip bleaches out the colour if it is caused by phenothiazines but not if due to salicylate. The tablet can also be identified by a spot test, giving a purple colour with ferric chloride or a green colour with cupric nitrate.
When methyl salicylate has been taken, the characteristic smell of oil of Winter Green may be detected on the breath. Diarrhoea is often present as well as vomiting. In children, the levels of salicylate necessary for intoxication are lower than in adults and plasma levels are greater than 30 mg/100 ml may produce moderate poisoning.
How does toxicity correlate with salicylate serum level? How reliable is the first serum salicylate value?
Because there are numerous reports of patients with a presumably benign initial salicylate determination who deteriorated thereafter, repeat testing of serum salicylate levels is mandatory at least 6 hours after ingestion. Once a peak level has been reached, levels should again be obtained in several hours. In the seriously ill patient, more frequent determinations may be necessary to assess efficacy of treatment and possible need for hemodialysis.
In overdosage, peak serum levels may not be reached for 4 to 6 hours. The dosage form (effervescent, enteric-coated, etc.) often influences the absorption rate.
It should be remembered that although salicylates are normally rapidly absorbed, the level may nevertheless rise steadily for several hours after ingestion. This happens in part because salicylate-induced pylorospasm may retard absorption. In conjunction with clinical signs and symptoms, the plasma salicylate levels remain the best guideline to toxicity despite protein-binding abnormalities, urine and plasma pH variations, and the importance of delayed absorption.
How does one manage salicylate poisoning?
As with any form of poisoning, treatment of acute salicylate poisoning is directed toward:
 prevention of further absorption of the drug
prevention of further absorption of the drug enhancing its elimination and
enhancing its elimination and reducing its toxicity.
reducing its toxicity.
Treatment for acute oral ingestion is largely supportive. There is no specific antidote for salicylate intoxication. Generally, therapy involves removal of salicylate from the gastrointestinal tract, and the correction of metabolic acidosis, dehydration, hyperthermia, hyperglycemia, and hypokalemia. The decision as to how rigorous a treatment should be is governed by the history of ingestion. Unfortunately, this is usually complicated since, in children, the quantity ingested is seldom known, and, in adults, salicylate poisonings also involve a combination of other drugs as well.
Prevention of further absorption
If intoxication as developed as a result of percutaneous absorption, it is clearly imperative to clean the skin thoroughly and withhold further applications of salicylic acid ointment.
For patients with pure salicylate ingestion who are asymptomatic or mildly intoxicated, activated charcoal (1g/kg) should be administered with a cathartic. Some investigators have suggested that salicylates in overdosage inhibit gastric emptying and that gastric aspiration and lavage is useful up to 12 hours after ingestion. However, the evidence for this is unconvincing. It seems more appropriate to advise gastric emptying up to 4 hours post-ingestion. An obvious exception to this recommendation is the gastric retention of enteric-coated formulations, for which lavage is probably indicated up to 12 hours after ingestion. Lavage should be carried out using as much tepid water (in aliquots of 300 to 400 ml) as is necessary to produce a clear gastric effluent. The efficiency of this procedure may be increased by gentle massage over the left hypochondrium while it is in progress. Repetitive activated charcoal is indicated for all moderately to severely intoxicated patients as it effectively reduces the serum salicylate half-life but may contribute to dehydration and electrolyte disturbances. Enough cathartic to ensure passage of a charcoal stool is required.
Enhancing of elimination
A variety of techniques has been used in attempts to enhance the elimination of salicylate from the body and these include urinary alkalinization, hemodialysis and hemoperfusion.
Alkalinization in children poses a difficult problem as the excess inorganic production and accompanying aciduria may not be compensated for completely with bicarbonate administration. Alkalinization cannot be accomplished in children until all potassium deficiencies has been corrected.
Hemodialysis or hemoperfusion is recommended for any patient with initial blood salicylate levels of more than 160 mg/100 ml or a 6 hour level of more than 130 mg/100 ml, acidosis unresponsive to bicarbonate and volume, renal failure, persistent CNS manifestations, congestive heart failure, ARDS, or progressive deterioration after all other measures. Indications for hemodialysis are :
 patients with acute ingestion and serum levels higher than 120 mg/100 ml or with severe acidosis and other manifestations of intoxication.
patients with acute ingestion and serum levels higher than 120 mg/100 ml or with severe acidosis and other manifestations of intoxication. patients with chronic intoxication with serum levels higher than 60 mg/100 ml and any confusion or lethargy.
patients with chronic intoxication with serum levels higher than 60 mg/100 ml and any confusion or lethargy.
Hemodialysis permits control of electrolyte and fluid balance should signs of circulatory overload develop. Hemoperfusion is also very effective but does not correct acid-base or fluid disturbances.
Is activated charcoal effective?
Each gram of activated charcoal can adsorb approximately 550 mg of salicylic acid. It is effective with enteric-coated, sustained-release, and standard tablets. The sooner the activated charcoal is given subsequent to the ingestion, the better the adsorption. It appears that giving activated charcoal in a 10:1 ratio to salicylate ingested will lead to maximal efficiency. Repeated doses of activated charcoal are probably warranted in any serious overdose.
The value of activated charcoal declines rapidly as the time from ingestion increases. Nevertheless, plasma salicylate concentrations continue to increase in some patients despite gastric aspiration and lavage, and it is possible that activated charcoal left in the stomach after lavage might reduce subsequent absorption.
Dr Abas is an associate researcher of PRN
Poisoning Emergency/ Information
-
Mon-Fri8am-10pm
-
Sat, Sun & Public Holiday8am - 5pm
-
Telephone04 6536 999
-
Telegram chat

