
NATIONAL POISON CENTRE
UNIVERSITI SAINS MALAYSIA
What is PRN?
PRN is the National Poison Centre set up based on the services provided by the Integrated Drug and Poison Information Service (IDPIS), Universiti Sains Malaysia since 1982. IDPIS evolves from several research activities carried out towards strengthening the health care services, particularly in the areas of information technology and informatics. It is aimed at auditing of health information as well as upgrading and strengthening the handling of drug and poison information directed towards health and allied professionals as well as the lay public.
Why PRN?
To reduce the mortality, morbidity, cost and occurrence of poisoning in a manner that strives for excellence, compassion and innovation.
How does PRN meets the above mission?
By making available services so as:
- to reduce the risk and the occurrence of poisoning,
- to encourage optimum treatment of poisoning cases, by:
- providing information on poisons and advice in poisoning cases
- conducting research and documenting poisoning incidences
- coordinating and conducting poison awareness and prevention education, and
- carrying out analytical tests and interpretations of laboratory results.
What services are available?
They are four (4) types of services; two (2) of which are currently available, viz:
- Drug and Poison Information Services
- to function as the main resource centre in providing information on toxicity and risk of poisons.
- to assist in the management of poisoning cases.
- to systematically disseminate drug and poison information and advise through the use of efficient, reliable and cost-effective methods.
- Research and Documentation Services
- to compile information on poisonous substances on a continuous basis.
- to develop computer applications in order to facilitate information transfer and use by the Drug and Poison Information Services.
- to research into systematic collection of poisoning data and information through toxicovigilance and toxicokinetic studies.
- Poison Education and Prevention Services
- to conduct and coordinate a systematic and continuous education and prevention programs in the training of allied and health professionals and the public.
- to collaborate with governmental and non-governmental agencies as well as other related bodies in carrying out its activities.
- to train students of health sciences students in the area of toxicology and poisoning.
- Toxicology Laboratory Services
- to provide supportive services in the identification of poison so as to assist diagnosis, management and prognosis of the poisoning cases.
- to be involved in surveillance program on population at risk to poisons.
- to collaborate with the development of governmental and non-governmental agencies on analytical methods.
Who should use PRN?
- Health and allied professionals
- Members of the public
- Governmental and regulatory agencies
- Non-governmental organizations
- Mass media, and other related bodies
When and how to use the service of PRN?
For emergency calls, trained pharmacists and professionals will be assigned to provide the necessary information needed using the resources available at PRN. Depending on cases, follow-up call(s) will be made to ensure the delivery of PRN continued support and assistance.
When should PRN be consulted?
When faced with:
- Acute and chronic poisonings involving,
- drugs and medicals products
- pesticides.
- household and industrial chemicals.
- natural toxins.
- Misuse and abuse of medicines/drugs.
- Drug-related problems including adverse reactions.
Detailed information may also be obtained upon completion of more extensive research and consultation.
What is required when contacting PRN?
In cases of exposure to poisons, please be ready with the following information.
- Name, age and weight of the victim.
- Caller's telephone number (this is important for follow-up calls).
- Name of product involved - read one at a time, the information written on labels, if any.
- Quantity of substance involved (provide estimate as accurate as possible).
- Time of the incident.
- Signs and symptoms.
- First aids given.
How does PRN operate?
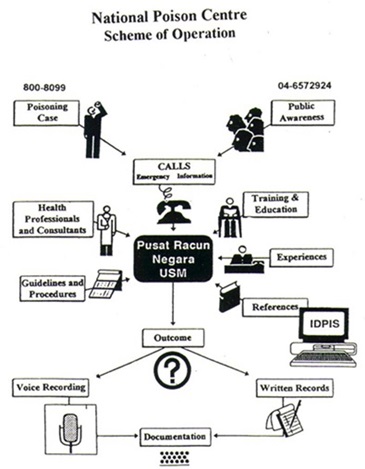
How to collaborate with PRN?
The Centre welcomes collaborative efforts as part of its enrichment program in areas pertaining to toxicologic and related research. Please contact the Director of the National Poison Centre, Universiti Sains Malaysia, 11800 Penang, Malaysia for further discussion.
Tel: 04-6570099
E.mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
| What to Expect When Calling PRN? |
In cases of actual poisoning exposure,
- the phone will be answered by a pharmacist or a scientist, who will be required to obtain a basic history and patient status report in order to provide better follow-up services.
- the pharmacists are trained and capable of interpreting literature and references in making recommendations relative to the hazards of a suspected poisoning, termination of the toxic exposure, symptomatic or supportive care, antidotal therapy, ancillary drug use, and indications for procedures like diuresis or dialysis.
- you will receive information that are condensed and concised for resources available at PRN, unless otherwise requested.
- all information is recorded on a specific report form (as enclosed) for ease of follow-up and continuity.
- when necessary you will be assisted in making arrangements for toxicological analyses, patient transfer, or obtaining specific consultations.
- as a matter of routine, in order to monitor the outcome and also to maintain accurate and complete records, one or more follow-up calls will be made to assess the patient's progress and obtain further history.
Your cooperation in all these are much appreciated. Thank you.
|
| PRN CONSULT |
 |
Review of Paracetamol Poisoning
Rahmat Awang, Pharm.D
Painkillers are generally available over-the-counter. The range includes aspirin, paracetamol and non-steroidal anti-inflammatory drugs. In children, paracetamol is the drugs of choice as an analgesic/antipyretic because it is relatively safe and easily accessible. In many similar instances it is also the preferred drug for adults. Unless properly used and stored such drugs can result in poisoning especially involving children. PRN CONSULT reviews some of the salient points in paracetamol poisoning. Paracetamol (acetaminophen, N-acetyl-p-aminophenol), is a major metabolite of phenacetin and acetanilide.
In some countries like the US, paracetamol accounted for 7% of the annual reported poisoning incidences and while in England, it is the leading cause of death from overdose, accounting for about 150 cases annually. Over the past few years, paracetamol has also been reported in some poisoning exposure in the country.
Should we be alarmed when somebody ingests an overdose of paracetamol?
Yes! Paracetamol is primarily hepatotoxic and may be fatal. However, if managed early, the likelihood of such consequences may be reduced.
An overdose of paracetamol can cause serious liver injury especially if the amount ingested is more than 140mg/kg in children or more than 6-7 gm in adults. Ingestion of more than 10gm (20 tablets of 500mg each) in adult may in fact be fatal. The risk for hepatotoxicity may even be increased if the patient also drinks alcohol, and/or on drugs that interacts with paracetamol such as barbiturates and other enzyme inducers.
How does the poisoning manifest itself?
Hepatotoxicity may be evident as late as day 4 from the time of ingestion. The manifestations associated with paracetamol poisoning may develop in 4 phases. Phase 1 which is between 1/2 to 24 hours post-ingestion may be seen with patient complaining of anorexia, nausea, vomiting and diaphoresis. Children however may look pale, quite ill and drowsy. These symptoms subside in phase 2, between day 1 and 3. Instead, patient may complain of right upper quadrant pain if there is any hepatic injury. Clinical laboratory tests when carried out may show elevation of SGPT, SGOT, LDH, bilirubin and prolongation of PT. Renal function may decline but BUN remains within normal limits. Following this, the patient may develop jaundice, coagulation defects and renal failure in day 3-5 (phase 3). Myocardial damage though has been reported is rare. Hepatic encephalopathy has also been observed. Nausea and vomiting may reappear. Biopsy may reveal centrilobular necrosis. Hepatic failure may result in death. If damage is reversible, complete resolution may occur in day 4 to week 2 (phase 4).
How can one predict the likelihood of hepatoxicity from paracetamol poisoning?
One approach which is simple but crude is to determine how much and how long ago was the drug taken. If the amount ingested is as stated above, then the likelihood is great, especially when the presence of signs and symptoms are evident. Patients admitted into the hospital should have their CBC, platelets, PT, bilirubin, BUN, electrolytes, glucose, SGPT, SGOT, alkaline phosphatase profiles checked.
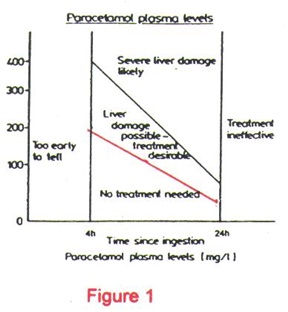
Another more reliable approach is to determine the paracetamol plasma level and then plotting this value onto the Rumack-Matthew Nomogram (Figure 1). The nomogram provides a good correlation between the paracetamol plasma level with the risk of hepatotoxicity. For example, a 4-hour paracetamol plasma level at various concentrations have been correlated with the following risk:
>300mcg/ml
250-300mcg/ml
150-250mcg/ml
120-150mcg/ml
< 120mcg/ml |
liver damage* and fatalities very likely
25% chance of developing acute renal failure
40% chance of developing liver damage*
25% chance of developing liver damage*
5% chance of developing liver damage*
relatively non-toxic |
*Liver damage is indicated by plasma SGOT or SGPT level of greater than 40
IU/L, a PT of 1.3 times normal and plasma bilirubin >1mg/dl.
It is therefore highly desirable to draw the paracetamol plasma level when the amount ingested is close to or more than the amount needed to cause hepatic injury. This holds true if the timing for plasma level determination is appropriate i.e. between 4 and 16 hours post-ingestion.
Another approach is to determine the half-life of paracetamol. Plasma half-life for paracetamol in normals is about 1-2 hours. If half-life is more than 4 hours, it may be suggestive of hepatotoxicity.
How does one uses the Rumack-Matthew Nomogram? What precautions does one have to take in interpreting the information?
The Rumack-Matthew Nomogram (Figure 1) estimates the potential for hepatotoxicity from paracetamol poisoning. In addition, it is also used to indicate the need for N-acetylcysteine (NAC) therapy. It is based on a semi-logarithmic plot of plasma paracetamol levels vs time. A plasma level that falls above the red line indicates a potential for toxicity and requires NAC treatment. Anyone who uses this nomogram however, has to understand that the nomogram is applicable only when the poisoning involves a single acute ingestion and that the blood level is drawn between 4 and 16 hours. Blood levels drawn before 4 hours may not represent peak levels since absorption is not complete and those drawn after 16 hours may not be predictive of potential hepatotoxicity or the need for NAC therapy.
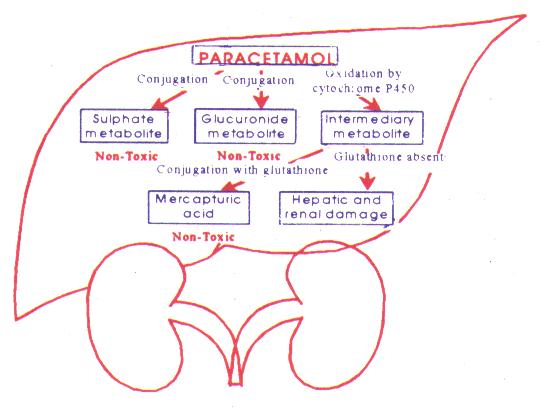
How does paracetamol result in hepatotoxicity?
Paracetamol is rapidly absorbed and metabolised by the liver primarily to sulfate and a glucuronide metabolites. Depletion of hepatic glutathione leads to the formation of toxic metabolites (? N-acetyl-benzoquinoneimine) which bind to hepatic macromolecules resulting in hepatic necrosis.
How does one manage paracetamol poisoning case?
In cases involving recent substantial ingestion, emesis is indicated unless the patient could rapidly become obtunded, comatose, convulsing. Emesis is most effective if initiated within 30 minutes. If available, syrup of ipecac at a dose of 30 ml in an adult or 15ml in a child of 1-12 years old is recommended. Charcoal administration with or without gastric lavage is also a preferred method of decontamination. However, its use may delay initiation of oral NAC since the antidote may be adsorbed by the charcoal rendering NAC ineffective.
NAC is an effective antidote for paracetamol poisoning when indicated. Dosing regimen for oral NAC is in the form of a 5% solution in a soft drink or juice starting with a loading dose of 140mg/kg followed by a maintenance dose of 70mg/kg every 4 hours for a total of 17 doses. IV administration of NAC can also be used.
The following situations warrant the use of NAC:
- if initial plasma paracetamol level is toxic on the nomogram. The therapy should be continued until the entire course is completed even if subsequent levels falls below the treatment line.
- if a toxic dose (7.5gms) is suspected to have been ingested within 16 hours of ingestion. Some Poison Centre recommend NAC therapy be given even up to 24 hours post-ingestion. Administration of NAC should never be delayed for lack of a paracetamol level. Loading dose should be administered but should be discontinued if level is below the nomogram treatment line.
- if paracetamol half-life is more than 4 hours.
Side-effects associated with the use of NAC include bronchospasm and anaphylaxis. Anaphylactic reactions may be life-threatening and thus treatment should be made readily available.
Selected Paracetamol Products:
- Algesic*
- Beamin/Junior*
- Benadryl C&F*
- Beserol*
- Biogesic*
- Calpol
- Codopar 118*
- Coritab*
- Decolgen*
- Dhamol
- Febricol*
- Fepril
- Horgesic*
|
- Junior Disprol
- MILI
- Milico*
- Naprex
- Norgesic*
- Nortes*
- Opigesic
- Panadeine*
- Panadol
- Panadol for Children
- Paradine*
- Parakol*
- Parclorphen*
|
- Parmol
- Partamol
- Redon
- Reno*
- Rhinitab II*
- Setamol
- Setramine*
- Setromol
- Sinutab*
- Sinuzin-D*
- Trangesic*
- Uphamol/Uphamol Jr
|
* Combination product containing Paracetamol
Source: DIMS Vol. 23 No. 3 1994
Technical Report on Paracetamol Poisoning will be
available from PRN soon.
Dr. Rahmat Awang is a Clinical Pharmacist at the PRN;
he heads the Drug and Poison Information Services.
NEXT ISSUE:
Organophosphate and Carbamate Poisoning |
Pusat Racun Negara, Universiti Sains Malaysia, 11800 Pulau Pinang






