1234567890
prn8099 - Number 4, September 1995


Unlike the industrialised countries, the major threat from pesticides in many developing countries come from acute poisoning. An estimate by the World Health Organisation (WHO) puts the annual number of severe poisonings at 3 million with about 220,000 deaths. However based on a survey of self-reported minor poisoning in four Asian countries, it was reported that each year 25 million agricultural workers in the Third World are exposed to pesticide poisoning.
In Malaysia, being an agricultural-based country, the use of pesticides is relatively prevalent. In Peninsular Malaysia alone there are about 1.5 million hectares of rubber and 0.6 million hectares of oil palm plantations accommodating almost 4.3 million people. The principal legislation for the control of pesticides in Malaysia is the Pesticides Act, 1974. The main aspect of this Act is to control manufacture and import of pesticides through registration. Other aspects of control include the licensing of premises selling and storing for sale of pesticides, labelling of pesticides and the control of import of unregistered pesticides for research and educational purposes.
Based on a number of limited studies, poisoning due to pesticides do occur. For example, surveys carried out by the local agro-chemical industry showed that in 1987, most of the estimated 715,000 rubber and oil palm smallholder farmers used paraquat. Over a 10 year period (1979-1988) pesticides accounted for 40.3% of the total cases (n=5,152) of human poisoning in Malaysia. Paraquat contributed 27.8%, other weedicides 1.7%, malathion 4.7%, other organophosphates 2.1%, organochlorines 2.6%, and other pesticides 1.4%. . It has been estimated that about 73 per cent of poisonings involving paraquat are suicidal, compared with 14% due to accidents and 1% due to occupational exposure. Annually 230 million ringgit are spent on weedicides alone.
Another survey showed that poisoning had occurred in 14.5% of the 4531 farmers growing vegetables, flowers and fruits in the Cameron Highlands. Hospital admissions showed that 32.1% were accidental pesticide poisoning while the other were 67.9 % suicidal cases.
In yet another study conducted in a predominantly agricultural area in 1991, it was reported that 12.2% out of a total 264 cases treated in a teaching hospital were due to pesticides. In a recent study, the serum pseudocholinesterase levels which was used as an indicator for exposure to the organophosphates, were found to be significantly lower in vegetable farmers; this reduction being dependent upon the length of exposure to these pesticides.
Thus, the use of pesticides in Malaysia can give rise to many serious concerns. A report by the Malaysian Factories and Machinery Department (1991), revealed that the accident rate for improper handling of pesticides is four times higher than that of other industries, and is as high as 93 per 1,000 workers as compared with the national average of 23 per 1,000. This seems to indicate that there is insufficient educational materials and information on safety and apparent lack of precaution in handling of pesticides. In Tanjung Karang, a paddy growing area, 72% of rice farmers experienced poisoning symptoms when handling pesticides and proper clothing, goggles, shoes and repiratory masks were seldom worn. In 1989, 448 pesticide workers received medical treatment at government hospitals. Another report in 1994 also highlighted the death of about 73 cattle suspected to be due to paraquat poisoning as a consequence of re-entry into the sprayed area.
Report of the Department of Chemistry Malaysia for 1989-1991 shows that 33.5% of human poisoning cases involved pesticides. Breakdown of the pesticides identified by the headquarters laboratory is shown in Figure 1:
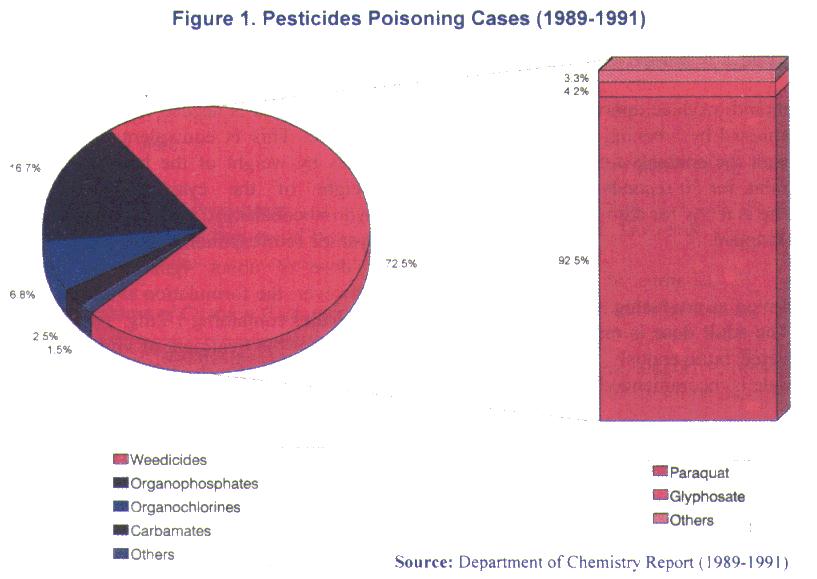
As for 1993, the cases of poisoning due to chemicals in the agricultural sector was reported as 528 with 80 deaths. All these figures are important as they provide some indications as to the nature of poisoning in the country. However, they do not give an accurate picture as to the extent of the problem that we face currently. Going by experiences of many developed countries, the 'penetrant rate' of poisoning cases is 6 per thousand population; if this is also true for the Malaysia then the figure is staggering. Inanother words, there is a large number of unreported cases that need to be uncovered. A more comprehensive and well-coordinated data collection system therefore need to be devised so that we are more informed of the problem at hand. The cooperation of all sectors is imperative if this is to be realised.
EMERGENCY DRUGS AGENTS USED IN THE TREATMENT OF POISONING
Compiled by Razak Lajis
There are a number of agents normally used in the treatment of poisoning. Use of a specific antidote is invaluable in treatment of a specific poison. These agents also counteract the effects of the poison by different mechanism of action. They could be in the form of chelating agents which normally form a nontoxic complex with the poison and is readily excreted. They can also act as physiologic antidote as in the case of atropine in organophosphate poisoning. Generally, before any of the drugs are used, the evidence for toxicity of the poison and indication of the drugs concerned should be considered together with the expected efficacy of the treatment. It is not recommended to used drugs that may further add to the toxic effect of the poison.
A readily available and practical guide to the agents or drugs used in the treatment of poisoning is important. The decision to keep individual items will normally depend on expected cases of poisoning which occur in the area. It is important, however, to be prepared for the unusual so far as it is reasonable.
|
PRN CONSULT 
Review of CYANIDE POISONING
Rahmat Awang, PharmD
Cyanide is one of the most rapidly acting and lethal poison known to man; with the hydrocyanic acid and its sodium and potassium salts being the most dangerous forms. The acid is extremely volatile, and has the ability to produce the deadly hydrogen cyanide gas, which has the distinctive odor of bitter almonds. Inhalation causes severe toxic effects that lead to death within minutes.
How can anyone be exposed to cyanide poisoning?
Cyanide poisoning can happen under various circumstances depending on the exposure to the types of cyanide sources. Cyanide intoxication can occur as a result of toxic gases produced by the pyrolysis of plastics or nitrile-based polymer fibers. It can also be the result of administration of certain drugs (e.g., sodium nitroprusside and laetrile), from ingestion of plants containing cyanogenic glycosides (e.g. cassava) or inhalation from industrial or occupational causes (e.g. electroplating). Cyanide poisoning can also results from exposure to aliphatic nitrile compounds (e.g. acetonitrile) or dermal/ingestion routes of cyanide salts and aliphatic nitriles. The table below provides examples of sources of cyanide.
Table 1. Types of Cyanide Sources
| Sources | Types |
|---|---|
| Gaseous form | Cyanide gas from fumigation of insects and rodents, cigarette smoking, production of combustion of petrochemical, products of combustion in fires, especially involving plastics, wool, silk, synthetic rubber, polyurethanes and asphalt. |
| Liquid and solid forms | Cyanide salts and solution containing these salts. Byproducts of industries such as petroleum refining, ore extraction, electroplating, metal heat treating. |
| Medicines | There are medicines that can release cyanide. Some examples include:
|
| Plants | Prunus species - Peach, plum, apricot, bitter almond. Amygladin is present in the leaves,flowers, barks and seeds. Sorghum species - Johnson grass, sorghum, sudan grass, arrow grass Linum species - Flax, yellow pine flax Pear, apple, crabapple seeds Bamboo, cassava (tapioca), linseeds |
How is cyanide formed and how does it affect our body?
Ingestion of cyanogenic plants, laetrile or a nitrile compound have a prolonged delay, up to 12 hours before the development of symptoms, since time is needed for the amygladin to be converted to cyanide through a metabolic reaction as depicted below:
| C20H27NO11 amygladin |
+ | 2H20 water |
-> | 2C6H12O6 glucose |
+ | C6H5CHO benzaldehyde |
+ | HCN hydrocyanic acid |
Amygladin is usually found in leaves (wilted or fresh), flowers, barks and seeds of some trees. When the seeds are crushed and the pulp moistened, an enzyme emulsin will be released before reacting with amygladin. Some plants are believed to contain large amount of amygladin.
Similarly, with the nitrile compounds, time is needed for absorption and metabolism of the nitrile compound to cyanide.
In contrast the effect from inhalation of cyanide gas is immediate. Patients exposed to high concentration of cyanide gas often become unresponsive within seconds and may die in less than one minute if no supportive care is instituted. A concentration of 0.2 to 0.3 mg/liter is almost immediately fatal, while 0.13 mg/liter (130ppm) is fatal in about an hour.
Dermal exposure rarely leads to toxic effects unless a large surface area is involved. Nitrile compounds are more readily absorbed through the skin, and their toxicities are likewise being delayed.
Patient receiving nitroprusside is also at risk of developing cyanide toxicity, particularly with long-term infusions. Cyanide is an intermediate metabolite of nitroprusside metabolism, while the thiocyanate which is approximately 100 times less toxic than cyanide is the final metabolite. Once the thiocyanate is formed, it is slowly excreted by the kidney. When nitroprusside is administered into the body, the normal tissue rhodanese and endogenous thiosulfate irreversibly converts the cyanide ions to thiocyanate. A state of malnutrition, surgery and diuretic administration can lower the body stores of thiosulfate. Cyanide levels begin to rise when stores of thiosulfate are depleted and, methemoglobin is saturated. Free cyanide binds and inactivates the ferric containing enzyme, cytochrome oxidase, found in the mitochondria of cells. This will lead to tissue anoxia and anerobic metabolism.
Though cyanide toxicity is said to be uncommon, 142 case reports involving 25 fatalities have been documented by the FDA Spontaneous Reporting System.
What are the signs and symptoms of cyanide poisoning?
Cyanide is readily attracted to many enzymes that has a metallic component such as the cytochrome oxidase, nitrate reductase, myoglobin, ribulose diphosphate carboxylase, and the catalase. Binding to the cytochrome oxidase, a mitochondrial enzyme responsible for cellular respiration will result in the inhibition of aerobic metabolism and consequent histotoxic anoxia state.
Unconsciousness, dyspnea and cyanosis are the three most frequently reported signs and symptoms of cyanide poisoning. The range of toxic effects that could be expected in cases of cyanide poisoning is shown in the table below.
Table 2. Signs and Symptoms of Cyanide Poisoning
| Systems | Manifestations |
|---|---|
| CNS Disturbances | Headache, anxiety, disorientation, lethargy, seizures, coma, cerebral death. |
| Cardiovascular Instability | Hypertension -> hypotension, tachycardia -> bradycardia, ST-T wave changes, dysrrhythmias, AV block, cardiovascular collapse |
| Changes In Oxygenation | Tachypnea -> apnea, venous hyperoxemia: red venous blood, increased mixed venous O2 content (SvO2), decreased O2 consumption (VO2), narrow arteriovenous oxygen difference (AvO2diff), brick-red skin (occasional cyanosis) |
| Metabolic Acidosis | pH - elevated blood lactate and/or elevated lactate: pyruvate ratio |
| Others | Nausea, vomiting, abdominal pain, increased salivation. |
Hypoxic signs in the absence of cyanosis may be considered to be a diagnostic clue, but cyanosis may occur as a late sign as described in most clinical cases of cyanide poisoning
How can cyanide poisoning be confirmed?
Cyanide poisoning should be suspected based on the patient's history (such as patient's occupation, location of exposure, premorbid mental status) and the sudden onset of symptoms. The presence of a bitter almond odour is highly diagnostic. However, as many as 20 to 40% of persons are not able to sense the smell. The presenting signs and symptoms such as altered mental status, unexplained anion gap metabolic acidosis and tachypnea in the absence of cyanosis with blood, that is bright red in colour, is also highly indicative of cyanide poisoning.
Determination of cyanide levels may be one way of confirming cyanide poisoning. Correlation between whole blood cyanide level and symptomatology have been established. The following blood level seems to corresponds quite well with the following signs and symptoms.
| LEVEL mcg/ml | SYMPTOMATOLOGY |
|---|---|
| <0.03 | Normal |
| 0.5-1.0 | Hyperventilation, tachycardia |
| 1.0-3.0 | Decreased mental state, may be fatal |
| >3.0 | Fatal unless treated. |
Though determination of cyanide levels are of great use, it should be realise that blood level may not reflect the dynamic process unique to a person's threshold for toxicity and that in an emergency, decision to treat therapeutic cannot wait for the laboratory results which may take hours.
How is cyanide poisoning managed?
Cyanide poisoning is a true emergency. Death may occur within minutes. However, there is still chance to keep the patient alive and the longer the patient is kept alive, the better the chances of recovery. The approach of treating patient with cyanide poisoning can be grouped into two main areas:
- general supportive measures
- specific antidotal therapy.
In a life-threatening situation, the following general supportive measures are instituted quickly:
- Establish airway and intubate patient if necessary. This is important because respiratory arrest usually develops quite rapidly. In some, assisted ventilation may be required. Administration of 100% oxygen may also be required even if the partial oxygen pressure (pO2) is normal.
- With inhalation exposure, remove the patient from the surrounding atmosphere in which absorption is taking place. With dermal exposure, remove contaminated clothing and wash skin thoroughly with soap and water. With ingestion of alkaline cyanide salts, attempts should be made to prevent gastrointestinal absorption with the use of activated charcoal.
- Correct acid-base imbalance by administering sodium bicarbonate if pH is < 7.16 and patient not responding to antidote therapy.
- Administer normal saline to provide volume support to maintain blood pressure. Pressor support such as dopamine hydrochloride may also be required if necessary.
- Management of seizures, arrhythmias should be instituted quickly if these happen.
What are the antidotes and how do they work?
Many different approaches to treatment of cyanide intoxication have been proposed. This includes the use of methemoglobin generators, cyanide complexing agents, and sulphur donors. Most of the present concepts of therapeutic intervention were developed 50-150 years ago.
The antidote kit known as the 'Eli Lilly Cyanide Antidote Kit' consisting of (a) amyl nitrite inhalants (12 pearls of 0.3mls each), (b) sodium nitrite (2 amps. of 300mg in 10mls each) and (c) thiosulfate (2 amps. of 12.5g in 50mls each) has been the primary antidote used for cyanide poisoning in the United States since the 1930s.
Its use is directed at reducing the amount of free cyanide that can bind to cytochrome oxidase and at releasing cyanide already bound. This is accomplished by administering of sodium nitrite (300mg for an adult) to produce methemoglobin, which competes with cytochrome oxidase for free cyanide, and administration of thiosulfate, which enhances the biotransformation of cyanide to thiocyanate, a less toxic compound.
The use of amyl nitrites should produce approximately 5% methemoglobin. This is achieved by breaking the pearl and allowing the patient to breath the contents from a cloth, or through intake valve of Ambu, for 30 seconds of each pearl used. Once the sodium nitrite is ready for administration, the use of amyl nitrites can be stopped.
The use of 600mg sodium nitrite (2 amps. of 100ml of 3% w/v x 10ml = 300mg) is aimed at producing approximately 25-30% methemoglobin. The adult dose is one ampoule or 300mg sodium nitrite, injected intravenously. For children, the following dosage schedule is recommended:
| Hemoglobin Level | Initial Dose of 3% Sodium nitrite | Initial Dose of Sodium thiosulfate |
|---|---|---|
| 7 gm/dl | 0.19 ml/kg | 0.95 ml/kg |
| 8 gm/dl | 0.22 ml/kg | 1.10 ml/kg |
| 9 gm/dl | 0.25 ml/kg | 1.25 ml/kg |
| 10 gm/dl | 0.27 ml/kg | 1.35 ml/kg |
| 11 gm/dl | 0.30 ml/kg | 1.50 ml/kg |
| 12 gm/dl | 0.33 ml/kg | 1.65 ml/kg |
| 13 gm/dl | 0.36 ml/kg | 1.80 ml/kg |
| 14 gm/dl | 0.39 ml/kg | 1.95 ml/kg |
Before use, the sodium nitrite is mixed in an IV drip and administered slowly over about 20 minutes. The blood pressure should be monitored since sodium nitrite has a significant vasodilating effects and may cause severe drop in blood pressure if administered rapidly. If symptoms persist, a second dose of sodium nitrite may be needed. This is accomplished by administering half the first dose 30 minutes later.
Upon completion of the sodium nitrite infusion, 12.5mg of sodium thiosulphate may begin (1 ampoule= 50 ml of a 25% w/v solution) for an adult and 1.65 ml/kg for children.
One of the disadvantages in using the Eli Lilly Cyanide Antidote Kit is that the methemoglobinemia may reduce the oxygen carrying capacity of the blood. Fatal methemoglobin have been reported with excessive administration of sodium nitrite. Under such circumstances, exchange transfusion is the preferred treatment modality. It is not advisable to give methylene blue since methemoglobin may release cyanide ions from the cyanmethemoglobin.
What are the other antidotes available for cyanide poisoning?
- Hydroxocobalamin (Vitamin B12a)
Hydroxocobalamin is said to be one of the most promising antidote available. The advantage using hydroxocobalamin is the lack of adverse effects seen with the nitrites such as methemoglobinemia and hypotension. It works by exchanging the hydroxy group for cyanide to form the non-toxic cyanocobalamin (vitamin B12). One molecule of vitamin B12a is needed to detoxify one molecule of cyanide. This is equivalent to a dose of about 50 times by weight of the hydroxycobalamin for each weight of the cyanide. Thus, about 1400mg hydroxocobalamin is required to detoxify 1 mmol of cyanide (corresponding to 65mg of KCN). Currently, a dose of about 4gm is employed in Europe. However, the formulation available in Malaysia is in ampoules containing 1-2mg. This formulation is only useful for the treatment of vitamin B12 deficiency. It is not suitable for the treatment of cyanide poisoning because too many ampoules are required. Hydroxocobalamine formulation that is used for this purpose contains 4mg of hydroxocobalamin powder and is available in France. Prior to use, the powder is reconstituted with 80ml of a 10% thiosulphate solution. This will reduce the total amount of hydroxocobalamin.
Most common side-effects observed with the use of hydroxocobalamin include an orange/red discoloration of the skin, mucous membranes and urine lasting about 12 hours. These however are not of much concern. - Dimethylaminophenol (DMAP)
DMAP is another type of methemoglobinemia-forming antidote. It was designed to produce more rapid methemoglobinemia compared to sodium nitrite which takes about 12 minutes to induce significant methemoglobinemia. The main problem however, is the difficulty encountered in determining the optimum dose. Adjusting the dose based on clinical signs such as the degree of brown-cyanotic discoloration of the skin are often unreliable and in fact very misleading since the same feature can be achieved by very low concentrations of methemoglobin. This often leads to administration of too low doses of DMAP. - Dicobalt edetate (EDTA)
Dicobalt edetate is another effective antidote and does not produce problems associated with methemoglobinemia. It acts by chelating cyanide ions to form cobalt cyanide and monocobalt edetate. One concern however is that if it is administered to patient misdiagnosed as having cyanide poisoning, the patient may develop serious reactions.
Poisoning Emergency/ Information
-
Mon-Fri8am-10pm
-
Sat, Sun & Public Holiday8am - 5pm
-
Telephone04 6536 999
-
Telegram chat

