1234567890
prn8099 - Number 5, Novembrt 1995

Inhaled Poisons
Rahmat Awang, Pharm D
Inhalation exposures represent one of the most important route of poisoning. The lung, by virtue of its large surface area and good blood supply, provides an excellent route for absorption and distribution of toxic gases. Besides causing irritation and injury to the lungs, inhalation of gases in some, may even lead to significant systemic poisoning involving vital organs such as the brain, kidney and liver. Since, inhaled gases have similar toxic action (such as nitrogen and methane) though diverse in its chemical properties, it seems appropriate that they be classified based on their nature of toxic action (see table below). Exposure to these substances however may produce varied effects. The nature, extent and severity of the effects to be observed may depend among other things; the types of gas involved, its solubility, concentration as well as the extent and duration of exposure. Poorly ventilated exposure site will very likely cause more significant effects especially in individuals who have preexisting lung diseases.
Gases and Vapours Classified According to the Nature of their Toxic Action
| Classes | Examples |
|---|---|
| Irritants | Ammonia, chlorine, suphur dioxide, ozone, phosgene, halogens, acrolein |
| Simple Asphyxiants | Nitrogen, hydrogen, methane, liquid petroleum gas, propane, carbon dioxide, dichlorotetraflouroethane |
| Chemical Asphyxiants | Carbon monoxide, hydrogen, cyanide, nitriles, hydrogen sulphides |
| Central nervous system depressants | Aliphatic hydrocarbons, chlorinated hydrocarbons, acetone, ethyl ether, benzene |
| Neurotoxic agents | Carbon disulphide, mercury, acrylamide, n-hexane, methyl n-butyl ketone |
| Hepatotoxic agents | Carbon tetrachloride, chloroform, allyl alcohol, bromobenzene |
| Nephrotoxic agents | Carbon tetrachloride, chloroform, trichloroethylene |
| Agents damaging blood | Nitrobenzene, arsine, naphthalene |
| Agents damaging bone marrow | Benzene, trinitrotoluene |
| Carcinogens | Vinyl chloride, 2-naphthylamine, bis(chloromethyl)ether |
A Malaysia-wide population-based incidence data on exposure to toxic gases is lacking. However, a report published in a local newspaper recently cited a total of 45 death from such exposure since 1992. This year alone, a number of gas related tragedies has been encountered. Two such tragedies were chlorine gas leak in Ipoh and possibly hydrogen sulphide exposure in Klang. The former occurred when a gas cylinder containing high concentration of chlorine was cut in a metal scrapyard. The released gas resulted in 32 admissions (including 11 children, five firemen and four policemen) to Ipoh Hospital. Luckily, all 32 patients responded well to treatment. In the latter incidence, 14 people were admitted to Tengku Ampuan Rahimah Hospital after being exposed to toxic fumes (believed to be hydrogen sulphide) from a drilling barge at West Port in Klang. From the fourteen admitted, four victims died while two others were critically ill. One thing to note is that in these tragedies, there seems to be a general lack of safety measures with complete disregard for occupational safety and health regulations.
PRN8099 wishes to highlight some of the harmful effects of gases that are commonly encountered under the categories of asphyxiants and irritants, so that there will be greater awareness in dealing with cases of inhaled poisons.
Asphyxiants
Simple asphyxiants are physiologically inert. When present in high concentration, they displace the oxygen in the atmosphere. Victims exposed to them will suffer from lack of oxygen. Carbon dioxide and methane are classic examples of simple asphyxiant.
Chemical asphyxiants on the other hand interfere with the body's ability to utilise oxygen. They either prevent oxygen delivery (such as carbon monoxide and hydrogen sulphide) or inhibit the utilization of oxygen by the cells (such as cyanide; see PRN8099, No.3, Sept. 1995).
Both types of asphyxiants however produce, if any, minimal direct injury to the respiratory system though they can cause marked neurologic and metabolic alterations.
Irritants
Irritants can be classified into two groups. They are the primary irritants that produce little systemic effects and secondary irritants that produce both respiratory and systemic toxicity. Toxic substances that are classified under secondary irritants include hydrogen sulphide, ozone, acetylene, methylated halogens and metal fumes.
Irritants have the ability to cause injury and induce inflammation of the mucous membranes upon contact. Individuals exposed to these gases may develop signs and symptoms that are either predominantly associated with upper or lower respiratory tract. These depend very much on the solubility of the gas. Highly water soluble gases like ammonia affect mainly the upper respiratory tract, while low water soluble gases like phosgene affects the lower respiratory tract.
Generally, irritation of the upper respiratory tract cause rhinitis, pharyngitis, cough and laryngeal edema, while irritation of the lower respiratory tract may cause pneumonitis, pulmonary edema, and hypovolemic shock. Signs of lower respiratory tract involvement may not necessarily be preceded by signs of upper respiratory tract involvement. However, injury to the upper respiratory tract may be extended to involve the lower respiratory tract if the exposure is prolonged. This seems to be the standard for gases with intermediate water solubility. Sulphur dioxide which belongs under this category generally cause upper respiratory tract injury in the beginning, then progressing to lower respiratory tract irritation when the exposure is prolonged.
Profiles of Some Common Irritants
Ammonia can be found in refrigerators, fertilisers, household cleaning and bleaching agents, and liniments. Products containing ammonia may be available in two forms, the 28% solution which is the industrial ammonia solution and the 5-10% solution which is the household ammonia. Ammonia has a strong penetrating odour and this can be detected at 5 ppm. The threshold limit value (TLV), which is the maximum tolerable concentration at an average of 8 hours workday without giving symptoms, is about 50 ppm.
Ammonia itself is not a systemic poison. The effects are mostly local, causing mild irritation to severe corrosion of the mucous membranes. At low concentration (400-700 ppm), the skin, eyes, nose, throat and lungs may become irritated immediately while at high concentration (>1000 ppm) the victim may experience violent coughing, sore throat, lacrimation, dyspnea, restlessness, blurred vision, pulmonary edema. At higher concentration between 5,000-10,000 ppm, the victim may lose consciousness, develop severe edema epiglottis, larynx and trachea, caustic burns of the skin/eyes, blindness and purulent bronchitis/pneumonia. The damage to the respiratory tract is the most serious and life-threatening consequence of ammonia gas exposure.
Chlorine is a yellowish-green gas with an irritating odour. It is used in the manufacture of plastics, as bleaching agents and for purifying water. Exposure to the gas usually follows a leak from a storage tank or at home as a result of accidentally mixing sodium hypochlorite (Clorox) with industrial acid cleaners, such as sodium acid sulphate.
The TLV for chlorine is 1 ppm. At low concentration (3-6 ppm), the gas can cause irritation of the skin, eyes, nose, throat and lungs. Nausea, vomiting, diaphoresis, headache, coughing, tightness of chest, redness/watery eyes, blurred vision, agitation, excitement, increase heart rate and blood pressure can also develop. At high concentration (>50 ppm) the gas is corrosive to the mucous membranes which may lead to pulmonary edema/pneumonitis, purulent bronchitis/ pneumonia, ulcerations and necrosis of skin and eyes. Both severe mucosal irritation and pulmonary parenchymal damage can occur within 10 minutes of exposure. At 1000 ppm, it is rapidly fatal. Victim may develop respiratory failure, coma, shock and death.
Sulphur dioxide is a by-product of gasoline combustion, oil refining and paper production. It is a colourless gas and upon contact with water at the mucosal membranes of the eyes and upper respiratory tract will form sulphuric acid that produces a severe irritation. Individuals exposed to the gas at concentrations between 10 to 15 ppm for 5 to 15 minutes may develop irritation of the eyes, nose, throat; rhinorrhea, choking, cough while exposure to high concentration may result in death. Prolonged exposure may lead to either irreversible bronchiolar obstruction or restrictive lung disease.
Hydrogen sulphide is a by-product of many industrial processes and decay of organic matter. It may be found in areas around petroleum refineries, tunnels and mines. It has a "rotten eggs" odour that is easily detected at concentration of 1 ppm. Exposure to the gas may cause irritation of the eyes and respiratory tract at low concentration (about 50 ppm) and respiratory paralysis and death at high concentration (about 1000 ppm). The TLV for this gas is 10 ppm.
Managing Inhalation Exposures
The best approach to managing inhalation exposure is prevention. We know that gas exposure may be encountered in a number of ways. In general these are occupational, accidental or intentional. In occupational exposure, the common cause of gas poisoning covers from carelessness or unawareness to potential danger from exposure, not reading and heeding labels, working in poorly ventilated areas and failure to detect malfunctions. In this instance, worker education, proper ventilation of work area and more stringent safety rules would help avoid accidental exposures. Managing the patient clinically generally involves removing the patient from the site of exposure without risking unnecessary exposure to other rescue personnel; airway or mouth to mouth resuscitation in patient having breathing difficulty and oxygen therapy.
PRN CONSULT 
Review of PARAQUAT Poisoning
Mohd Isa Abd Majid, PhD
Over the last ten years, paraquat has contributed to nearly a total of 700 poisoning cases in Malaysia. Out of these figures, it has been estimated that about 73% of the poisoning is due to suicide with the remainder as a result of accidental and occupational exposures. The reason for high incidence of paraquat as a suicidal agent is somewhat difficult to determine but effort has been made by the relevant agencies to reduce the number of registered products containing paraquat. The ingestion of commercial paraquat formulations in all suicidal cases is invariably fatal (about 60 % mortality rate) and the resulting clinical features can be seen in a time course of 3-4 weeks depending on the quantity of paraquat ingested.
What are the registered pesticide products containing paraquat in Malaysia and the content of paraquat in each product?
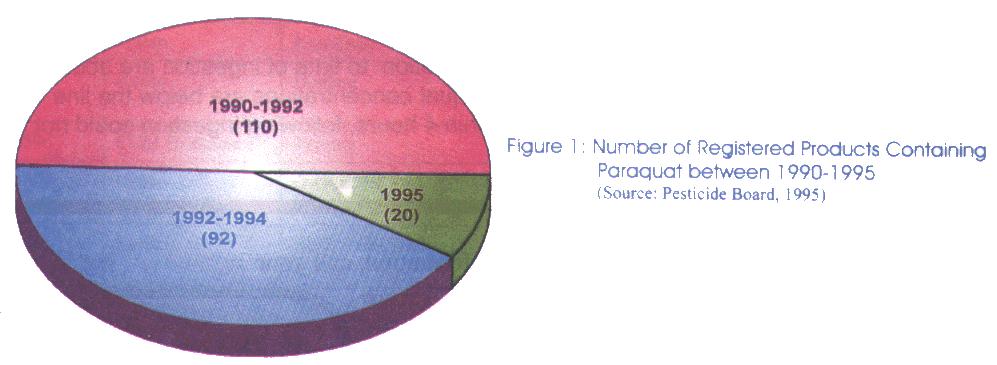
Commercial preparations of paraquat are normally sold in the form of liquid concentrate with a concentration ranging from 20% to 42% w/w. These solutions are available for agriculture use as dichloride salts which is water soluble. Besides being supplied in the form of a single active ingredient, there are products in the market containing paraquat in combination with other herbicides such as sodium chlorate and 2,4-dimethylamine. From 1992-1994, there were 92 registered products containing paraquat in Malaysia. The majority of these products were supplied to the farmers in the form of aqueous solution ranging from 13% to 25 % w/w. Besides the lower concentrated products, paraquat is also supplied in bulk, normally known as the technical grade, to large scale plantations. The normal concentration of paraquat for the technical grade products is 42% w/w. Following a reregistration exercise of pesticides product in 1995, currently there are about 20 products containing paraquat approved to be used in the agriculture sector. These products together with their content of paraquat are listed as below:
Table 1: List of registered pesticide products containing paraquat in Malaysia as of 1995.
| LRMP | Registrant | Trade Name | Active Ingredient (A.I) | A.I (%/W/W) | Formulation |
|---|---|---|---|---|---|
| 1249 | Halex | Weedway 253 | Paraquat Dichloride | 25 | Aqueous Solution |
| 1815 | Kenso | Ridweed | Paraquat Dichloride 2,4-D Dimethylamine |
16.6 28.0 |
Aqueous Solution |
| 1979 | Ancom | Anuron | Paraquat Dichloride Diuron |
24.6 17.9 |
Concentrate |
| 1992 | Serba Kimia | Double Action | 2,4-D Dimethylamine Paraquat Dichloride |
20.3 17.7 |
Aqueous Solution |
| 2422 | Kenso | Ken-Para 990 | Paraquat Dichloride | 19.0 | Aqueous Solution |
| 2484 | Kenso | Ken-Quat | Paraquat Dichloride | 25 | Aqueous Solution |
| 2486 | Kenso | Hentam | Paraquat Dichloride Sodium Chlorate |
16.5 11.0 |
Aqueous Solution |
| 2596 | CCM Bioscience | Terquat | Paraquat Dichloride | 19.0 | Aqueous Solution |
| 2621 | Kenso | O-Quat 96 | Paraquat Dichloride Sodium Chlorate |
16.5 11.0 |
Aqueous Solution |
| 2635 | Kenso | Buah Emas Haracol | Paraquat Dichloride Diuron |
24.6 17.9 |
Concentrate |
| 3099 | Imaspro | Pro-Col | Paraquat Dichloride Diuron |
24.65 17.86 |
Concentrate |
| 3783 | Zuellig | Goldquat Dcl 276 X'tra | Paraquat Dichloride | 25.0 | Aqueous Solution |
| 3789 | Agricultural | Clean Up 13% | Paraquat Dichloride | 13.0 | Aqueous Solution |
| 3819 | Agricultural | Clean Up 19% | Paraquat Dichloride | 19.0 | Aqueous Solution |
| 3820 | Farmcochem | P - Kill | Paraquat Dichloride | 22.0 | Aqueous Solution |
| 3821 | Serba Kimia | SK Hi Kill | Paraquat Dichloride | 24.0 | Aqueous Solution |
| 3822 | Serba Kimia | Par Kill | Paraquat Dichloride | 22 | Aqueous Solution |
| 4018 | Imaspro | Uniquat Technical | Paraquat Dichloride | 42.0 | Concentrate |
| 4021 | Halex | Halex Paraquat 19 | Paraquat Dichloride | 19 | Aqueous Solution |
| 4048 | Kenso | Kenmoxone | Paraquat Dichloride | 13.0 | Aqueous Solution |
(Source: Pesticide Board of Malaysia, 1995).
How Does Paraquat Act on the Weeds?
In terms of its action on the weeds, paraquat interferes with the intracellular electron transfer systems, thus inhibiting the reduction of NADP to NADPH during photosynthesis. This will then result in the accumulation of superoxide radical which causes destruction of lipid cell membranes.
What are the Clinical Manifestations of Paraquat Poisoning?
The symptoms of poisoning from paraquat depend on the amount and the route of absorption into the body. Exposure to paraquat through brief dermal contact or inhalation of spray mist produces mild symptoms consisting of local skin irritation, reversible irregularities in nail morphology and ocassional epistaxis. Following exposure to the eye, corneal injury, lacrimation and protracted opacification may result.
The major symptoms from paraquat ingestion are seen in the mouth and the oesophagus after ingestion of the concentrate and may include ulcers on the lips, burning and ulceration of the tongue and pharynx. In some massive ingestion cases, oesophageal ulceration may occur which can proceed to oesophageal perforation. Following ingestion of greater than 50 ml of the liquid concentrate, patient may develop pulmonary oedema, cardiac failure, renal failure (which may result within hours of ingestion), hepatic failure and also convulsions caused by central nervous system involvement. Under these circumstances, death may occur within several hours to a few days as a result of multiple organ failure.
Ingestion of a smaller volume (10-20 ml) of the concentrate produces the same symptoms with the exception that the development of renal failure occurs within 2 to 6 days after ingestion. Renal failure may be manifested by proteinuria and oliguria which then progresses to acute tubular necrosis. The major effect of poisoning at this volume is the accumulation of high concentrations of paraquat in the lung. In the lung, the paraquat ion undergoes a continuous reduction-oxidation process to form free radicals capable of reacting with oxygen. This reaction leads to the production of a reactive oxygen also known as superoxide anion and the regeneration of the paraquat ions. The superoxide anion is then converted into hydrogen peroxide by the enzyme superoxide dismutase. The continuous generation of the superoxide anion under the depletion of precursor NADPH and hydrogen peroxide will then attack the polyunsaturated lipids present in the lung membranes to produce lipid hydroperoxides ( a form of lipid free radical) which in turn can react with further unsaturated lipids to form more lipid free radicals thereby perpetuating the system. The resulting cellular membrane damage reduces the functional integrity of the lung cells, affects efficient gas transport and exchange, and induces respiratory impairment.
The development of pulmonary lesion as a result of cellular lipid peroxidation can be divided into two distinct phases. The first phase which occurs within 1-3 days after ingestion is shown by the destruction of the alveolar epitelial cells leading to alveolitis, characterised by the production of pulmonary oedema and infiltration of the air spaces of the lung and the interstitial tissue with neutrophil polymorphs. The second phase involves the development of extensive fibrosis as a response to the acute alveolitis occuring in the first phase. During this stage, the proliferation of the fibroblasts and deposition of collagen will further reduce the effectiveness of gaseous exchange. The consequence is death resulting from severe anoxia. In summary, as the lung function begins to deteriorate, the patient will suffer from breathlessness, tachypnoea, widespread crepitations and central cyanosis. This changes continue over a period of 5-7 days before the patient finally develops to respiratory failure.
What is the possible qualitative test for confirmation of paraquat poisoning and how can one determines its prognosis?
To confirm paraquat poisoning, a simple test is to check for the presence of paraquat in the urine. Normally, a 5 ml urine sample collected within a few hours of ingestion is made alkaline by adding 0.1 g of sodium bicarbonate. An equivalent amount of sodium dithionite is then added to the alkaline urine and the presence of a resulting blue colour indicates intake of a significant amount of paraquat into the body. A pale green colour may be obtained from a low concentration of paraquat. Since the absorption and renal excretion of paraquat are rapid, the failure to obtain a positive reaction from urine passed within 4 hours of the alleged ingestion can be interpreted as no significant quantity of paraquat ingested.
A suitable indicator to determine the prognosis of a paraquat poisoning case can be obtained from the nomogram by Proudfoot A T (1982) as shown in the Nomogram Chart. Based on the nomogram, the prognosis in an individual case can be predicted from the plasma paraquat concentration related to the time of ingestion. Patients whose levels fall below the line as shown in chart will almost certainly survive without treatment while those with levels well above the line are likely to die. Any patient whose plasma paraquat concentration exceeds 0.1 mg/l after 25 hours is predicted to be unlikely to survive.
How is paraquat poisoning managed?
There is NO specific antidote for paraquat poisoning. In such poisoning, treatment from confirmed ingestion of paraquat is largely supportive and aimed at interrupting the pathway for paraquat toxicity. Its management is primarily directed at removing paraquat from the site of absorption such as the gastrointestinal tract, increasing its excretion from blood and adopting measures aimed at preventing pulmonary damage. It should be noted that hospitalization is required in all cases of suspected paraquat poisoning.
Prevention of Absorption
Gastric lavage should be performed immediately if possible within 2 hours post-ingestion. This is to reduce further absorption of paraquat into the bloodsream. Gastric emptying should be initiated early as pharmacokinetic studies from poisoned victims showed that the plasma concentration in man rises rapidly to peak within approximately 2 hours of the ingestion of paraquat. Following gastric emptying, the administration of mineral absorbent such as Fuller's Earth, bentonite or activated charcoal is initiated to remove any unabsorbed paraquat remaining in the gastrointestinal tract. For activated charcoal, the usual dose is 30-100 gm in adult whereas the suggested dose for children is 1-2 gm/kg. Alternatively, the dose for Fuller's Earth and bentonite is 100-150 gm in the form of aqueous suspension in water for adult or 1-2 gm/kg for children. In some cases, cathartics for example magnesium sulphate, magnesium citrate or sorbitol, may be given every four hours either concurrently or separately with the absorbents.
Enhancement of Elimination
A variety of techniques has been used in an attempt to enhance the elimination of paraquat from the body. The recommended regimen for increased paraquat excretion from the blood is hemoperfusion. Hemoperfusion with the use of charcoal has been reported to reduce the majority of paraquat in the blood (by about 80%) and has been shown to increase the chance of survival among poisoned patient. Using this approach, the hemoperfusion was carried out for 8 hours a day and may be repeated until the paraquat level remained below 0.01 mg/ml. It is also important to measure the paraquat level in the blood during hemoperfusion to determine if paraquat has been released from the tissues necessitating further hemoperfusion. If the facility for hemoperfusion is not available, an alternative approach would be to hemodialyse the patient. In this case, however, paraquat has been reported to be not dialyzed very effectively. Despite this, hemodialysis should be started as early as possible and continued intensively during the first 24-48 hours so as to remove as much poison as possible from the body.
Prevention of Pulmonary Damage
Oxygen therapy: Paraquat is known to accumulate selectively in lung tissues and destruction of the lung tissues is exacerbated by the adminstration of oxygen. Animal studies which correspond to the same biochemical changes in human showed that the administration of 100 percent oxygen resulted in a significantly higher mortality rate when compared to those breathing room air. Thus it is suggested that the supplemental oxygen concentration in the inspired air to be not greater than 21 percent so as to maintain an arterial oxygen tension between 40-50 mmHg.
Drugs: Drugs such as steroids when given alone or in combination with immunosuppressants, colchicine, d-propranolol and superoxide dismutase have also been used to protect the lung through the reduction of fibrosis or displacing the paraquat from the lung. There is however no observed clear cut benefit unless they are given very early following paraquat ingestion.
Poisoning Emergency/ Information
-
Mon-Fri8am-10pm
-
Sat, Sun & Public Holiday8am - 5pm
-
Telephone04 6536 999
-
Telegram chat

