1234567890
prn8099 - Number 7, April 1996

PRN JOINS THE CYBERSPACE
Dzulkifli Abdul Razak
After working very closely with the print media since its inception, PRN is now turning to the electronic media in pursuing its outreach activities to the general public. Beginning in 1995, PRN have had the cooperation of three major dailies in providing regular columns on the various aspects of poisons information. The responses received from the readers of these columns have been very encouraging that soon it will be further extended to include two more dailies, the Berita Harian and Harian Metro which have shown interest to work with PRN under its enrichment programme. For that matter, PRN will always welcome collaboration with other responsible publishers, especially with the vernacular press.
PRN reaching out through the electronic media
While the relationships with the press continue to grow, PRN has decided to initiate a similar collaborative partnership with the electronic media for the year 1996. This is to complement its effort to further heighten the awareness of the general public especially those not well reached by the newspapers. This will increase the scope of its outreach to the various other segments of the population, especially children and those in the more remote areas of the country.
For a start, PRN began with a fortnightly talk-show on Radio 3 Pulau Pinang (Thursday, 2.00-2.30pm) to cover the northern areas. The aim of the show is to engage some discourses directly with members of the public on issues of current health interest, with emphasis on poisonings, drug use and pharmaceutical care. The first programme was aired on February 15, 1996 and it has been well-received since then. In the previous shows various issues were discussed "live", including any queries raised by listeners who called in. This provides additional avenues for the public to raise pertinent questions or clarify other points with specialists from the Centre.
PRN also scored another first in coming up with a one-minute 'television trailer' entitled "UBAT UNTUK ANDA BOLEH MERACUNI ANAK ANDA" - [Your medicine can poison your child]. The trailer a product of another collaborative effort between PRN and the Educational Technology and Media Centre (PTPM) Universiti Sains Malaysia was aired for the first time on TV1 prime-time, on March 2, with the courtesy of RTM. The trailer is a simulation of an incident depicting a careless mother who left an uncapped bottle of her medication unattended resulting in the poisoning of her own child - thus the message 'your medicine can poison your child'. This message have been aired repeatedly nationwide over the TV network on several occasions until today.
This trailer was introduced as part of an awareness campaign on the part of the Centre to bring to the attention of parents that medicines unless proper handled and used, can result in poisonings. This is particularly so when involving children under the age of 5 years as emphasised in the trailer. It is hope that with the support given by RTM, this important message could reach more homes and touch the hearts of many more parents is assisting PRN in making Malaysia a safer place to live in. In the same spirit, PRN calls upon other TV channels in the country to provide similar assistance in spreading the same message as an invaluable social service to their viewers. Such a collaborative effort is imperative if we are to collectively secure a better health status for all Malaysians, particularly those in the far away places only accessible through the TV transmission. Based on the responses generated by the trailer, PRN is planning to do another trailer focusing on yet another problem of poisoning as documented by the Centre.
Notwithstanding this, PRN in its second year of service, strategised to enhance its capability via the Cyberspace. The aim of going into the Cyberspace is to further enrich its outreach by optimising the use of today's technology. This is also in keeping with the expanding infocommunications in matters dealing with drugs, hazardous chemicals and poisonings of various types - food, natural toxins as well as herbal preparations. In fact, since the beginning of its operation last year, PRN has received a myriad of queries implicating all these substances. As such it is only appropriate that PRN begin to enlarge as well as enrich its experience by internetworking with other information sources around the world. At the same time this will enable PRN to offer its own experiences and expertise to the rest of the world in this era of 'information superhighway'. Indeed, PRN has been actively consulted many a times about various aspects of drug and poison information via the Internet from within Malaysia as well as internationally from as far as the Latin America.
Hence, since last year PRN has been kept busy diligently developing its own homepage headed by Puan Kamilah Beran, a system analyst and the webmaster. After much planning and teamwork, PRN is now ready to share part of the Cyberspace, and fulfilling the new promise of information technology to all Malaysians. Called the MALAYSIAN DRUG & POISON NET, it is the homepage of the Pusat Racun Negara (PRN). The Net will also be accessed through the USMNET under the subheading 'National Poison Centre'. The PRN homepage is scheduled for a soft launching on April 19, 1996 making it the first such network in this country and also the region. This is yet another milestone for PRN in trying to live up its mission: "To reduce the mortality, morbidity, accident and cost of poisoning with emphasis on excellence, compassion and innovation".
What is MALAYSIAN DRUG & POISON NET?
The Malaysian Drug & Poison Net (PRN-Net, for short) is part of the global electronic web connecting computers all over the world. It is the third homepage on health to be registered with Joint Advanced Research Integrated Networking (Jaring) operated by the Malaysian Institute of Microelectronic Systems (MIMOS).
The PRN-Net is operated by the National Poison Centre, Universiti Sains Malaysia. The interactive version could be accessed under the section: ABOUT THE NET (see below).
Why PRN-Net?
In the Nineties, the spread of computer communication has increased by leaps and bounds with Internet being one of the most popular network that is fast changing the world. In Malaysia, its usage is booming at an estimated rate of 20 per cent a month.
At the same time, the profile of computer users are also changing with the significant availability of personal computers at all levels of the society, and schools in the near future. Computers too set the character of many workplaces, places of learning and even homes. Although the diffusion rate of Internet among the general public is still low, PRN recognises that this is the way forward and to contribute in creating an information-rich Malaysia. It is with this in mind that PRN embarked on the development of the Malaysian Drug & Poison Net to further complement its existing services to the Malaysian public.
What is the mission and objectives of the PRN-Net?
The mission of the Malaysian Drug & Poison Net is:
"to promote health in an information society and to provide information in a healthy society".
Based on this the objectives of the PRN-Net are:
- to be aligned with the mission of PRN by maintaining a dynamic informative environment,
- to collaborate closely via the Internet in complementing all the services offered by PRN,
- to create awareness by making accessible to all a network of selected information sources globally, and
- to provide national, regional as well as global gateways in health-related matters with emphasis on drugs and poisons.
The PRN-Net is envisioned to evolve into a global WWW site for information on issues related to poisoning in particular, and health in general, in Malaysia.
How can PRN-Net be accessed and for whom?
It can be accessed in one of three ways:
- directly via the address: http://prn.usm.my;
- via USMNET under R&D and Consultancy
- via Jaring under Malaysian WorldWide site, Health & Medicine
It is open to all Internet users, particularly practitioners, educators as well as the public interested in the subject.
What are the advantages of PRN-Net?
The PRN-Net provides an up-to-date information not only about the activities of PRN but also on poison-related issues in Malaysia, and also around the world. PRN-Net also provides several unique and indigenous databases in the context of Malaysia. Currently it is also developing a section which carries information in the Malay language. In addition it also is a medium for continuing education in Clinical Toxicology for health professionals.
The PRN-Net also serves as a global gateway to more than 50 related WWW sites throughout the world. This will enable Malaysians to access these sites with relative ease and speed. In this sense it is also economical and reliable. Moreover, these sites that have been evaluated by PRN as dependable and of relevance to the field of toxicology and in times of emergencies and disasters involving hazardous substances. PRN-Net is available 24-hours all the year round.
What are the topics of interest available in PRN-Net?
There are 10 topics of interest, most of which are regularly updated. These includes:
- The Poisoned World -- This section represents a cumulative record of major poison-related events happening around the world. It is aimed at highlighting the fact that the "mother earth" is in an ailing state, and her inhabitants are being insidiously poisoned, looking back at all the 'toxic' events that unfolded in the various parts of the world, just last year. Under this section one can access information about the events occurring in all the regions (Africa, America, Asia, Europe and Middle-East); and indeed by the individual country in each of the region. In 1995, more than 15 countries have been listed, and to date another 5 have been added. It is updated monthly and allows for contributions world-wide from any Internet user.
- Traditional Malay Poisons -- This is the latest addition to the PRN-Net and is now under construction. It is aimed at providing a comprehensive data as well as information regarding indigenous poisons in Malaysia, especially of natural origins, and other infamous concoctions, charms and magic. To date it provides for an alphabetical listing of:
- Poisonous Plants (consisting of about 100 names, with one sample page),
- Venomous Snakes (consisting of about 50 names, with one sample page) and
- Santau - a poisonous concoction.
Graphical and pictorial representations which are being prepared currently based on research and documentation activities of the Centre. Two sample pages are included for viewing. More will be put in due time. A list of classical and modern references are also being prepared so as to allow interested parties to access more information on the subject.
- About the Net -- This is an electronic version of what was explained above, except that it is interactive, being linked to the various sections of the PRN-Net.
- PRN Emergency & First Aid -- This section provides information about the addresses as well as the other contacts necessary during cases of emergencies. It is also linked to databases related to First-aid in a number of poisoning situations as a means of quick reference.
- PRN Bulletins & Articles -- This section represents the electronic version of the all publications that originated from PRN, including an up-to-date compilation of all articles found in the bulletins of the Centre (PRN8099 & PenawaRacun) as well as those in the other printed media. This particular issue of PRN8099 is already being edited into the PRN-Net prior to its release in the printed format. The section keeps all articles archived for the year 1995, and to date, more than 150 articles could be accessed electronically via PRN-Net.
- News & Headlines (now renamed News & Health Info) -- The section on News & Headlines was started in October 1995 in the effort to capture all poisoning- and health-related news reported in the Malaysian dailies, and at times other sources within the region. The news items are listed weekly for each month, and a summary of each items are also provided, together with their reference sources. This is to enable users to gain access to the original news clippings that are kept under separate computerised databases in the Centre. The aim of this section is to provide some insights of the events happening in the country on a day-to-day basis. It serves as a reminder to Malaysians as to the 'state-of-poisoning' in the country at the end of each week. International health-related news (including poisonings), notably that provided by New York Times Your Health Daily and Reuters Health Information Services, are also linked to this section. Linked too, are selected health-related news WWW sites found in the Internet.
- Continuing Education & Meetings -- This section introduces the electronic version of Continuing Education on Clinical Toxicology (CECT) programme introduced through the professional bulletin of the Centre, PRN8099, since last year. Also enlisted are additional educational programmes on toxicology and pharmacological emergencies provided by other WWW sites in the Internet. Another feature in this section is the link to a number of WWW sites which list a variety of international conferences and scientific events and meetings that are to be held as far as the year 2000. This would be of interest to researchers and scientists who are keen to keep abreast with the knowledge frontier in toxicology and other related fields, and could facilitate their attendance and participation in such events. Some of these events listed are even conducted electronically.
- PRN Consult -- Under this section, reviews of various poisonous substance are placed, aimed primarily to assist practitioners in the field. These reviews include discussions on the management and treatment in cases of such poisonings, as well as aspects of basic toxicology. Each review provides a quick reference guide -ELCA- based on the appropriateness to carry out Emesis, Lavage, Charcoal treatment and Antidotal support. To date, 8 substances - drugs and non-drugs - have been reviewed and edited into the PRN-Net. More will be added every two monthly. The reviews in this section are also used as a basis for the CECT programme described above.
- PRN Links -- This a 'global gateway' especially dedicated as a link to the other WWW sites of significant interest to PRN that are available in the Internet. Its compilation is aimed at increasing the speed of information sharing and exchange in carrying its activities. PRN has endeavoured to list only selected WWW sites under the various category of Chemicals, Drugs, First-Aids, Food, Hazmats, and Poisons. These will be further expanded to include Natural Toxins and Poisonous Plants, and is up-dated from time to time. To date, more than 50 major WWW sites have listed and this could benefit the other users of Internet interested in such matters, especially in management of crisis.
- PRN Index -- This section provides an index listing of all the articles published by PRN in 1995. It is an electronic version of PRN Index 1995 and will be updated annually. It is aimed at facilitating users to look for the relevant materials, via the Internet, published by PRN. The index is based on the Library of Congress Classification Systems, 18th edition, 1995.
PRN CONSULT 
Review of Rodenticide Poisonings
Rahmat Awang, PharmD
Introduction
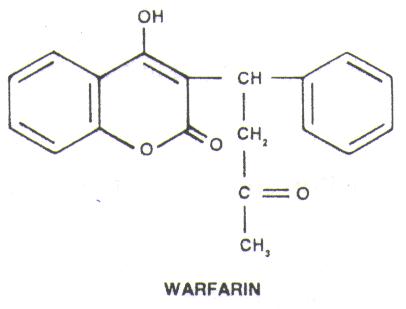
Rodenticide by definition refers to any product that is made commercially available for the purpose of killing rodents and other small pests. The chemicals used range from the highly toxic ones with the ability to kill on ingestion of a one-time dose, to a less toxic ones requiring repeated ingestions over a period of time.
Strychnine was the first agent employed for such purpose while thallium, sodium monofluoroactetate, arsenic oxide, zinc phosphide, yellow phosphorus, alpha-naphthyl-thiourea, red squill and norbormide were introduced much later. Because of their toxic potential, most of these agents are not of much use today, and they are largely replaced by the anticoagulant, warfarin. But, as more and more rodents begin to develop resistance against warfarin, a more potent second-generation warfarins were developed. These are long-acting anticoagulants (LAAs) and they are often referred to as superwarfarins.
All of these rodenticides are not ideal. Many can produce their toxic or lethal effects in humans mainly by ingestion of a large enough single dose. Their single-dose effectiveness that were considered advantageous in killing rodents make th em more dangerous in inadvertent or deliberate human ingestions. This should be of concern because as the number of commercial and residential of rodenticides increase so may the incidences of their accidental ingestions.
In this issue of PRN8099, various rodenticides will be reviewed with emphasis on the class of anticoagulants.
ANTICOAGULANT RODENTICIDES
What are anticoagulant rodenticides?
The anticoagulant rodenticides belong to a group that exerts a blood-thinning effects. It is used for many decades and are readily available as over-the-counter pr eparations. Generally, it consists of the hydroxycoumarin derivatives and indanediones. The 4-hydroxycoumarin compounds are made up of warfarin, difenacoum, bromadiolone and brodifacoum while the indandiones cover a wide range of chemicals including dipha cinone, pindone and chlorphacinone. Aside from warfarin, the others are said to be longer-acting. They produce a more potent and prolonged anticoagulant effect. The potency of these compounds is evident from brodifacoum's half-life of up to 487 hours, and its ability to inhibit the 2,3-epoxide of vitamin K1 for up to 3 years. This contrasts with warfarin's half-life of 37 hours.
What anticoagulant rodenticide products are available in Malaysia?
At present, there are altogether about 53 rodenticide products registered in the Malaysian market. Among these, 46 are anticoagulant rodenticides while another 7 are the zinc phosphide rodenticides. Of the 46 anticoagulant rodenticides, 25 contain warfari n while the remaining contain LAAs. The table below provide a breakdown of trade products according to active ingredients:
| Warfarin | |
|---|---|
|
|
| Brodifacoum | |
|
|
| Bromadiolone | |
|
|
| Chlorophacinone | |
|
|
| Coumatetralyl | |
|
|
| Diphacinone | |
|
|
| Stratagem | |
|
|
How do anticoagulants work as rodenticides?
As we all know, anticoagulant exerts its effect by inducing a coagulopathic state that would enhance bleeding tendencies. This is achieved by inhibiting the synthesis of the vitamin K dependent factors, namely, factors II, VII, IX and X. Through this mechanistic pathway, only the synthesis of new factors are affected and the increase in the prothrombin time will occur until currently circulating factors have been degraded. Thus, the anticoagulant effect is expected to be delayed; the first evidence may only to be seen as early as 8-12 hours postingestion. This is consistent with the half-life of factor VII of about 6 hours, which is the shortest among the vitamin K-dependent factor. The effects tend to peak in 1-3 days (coinciding with the 24-60 hours half-life of the other vitamin K-dependent factors) with the duration extending up to 5-7 days for warfarin. With the superwarfarin rodenticides, this effect is even more significant and anticoagulation may persist for weeks to months.
What are the clinical manifestations?
Haemorrhage is the most frequently encountered complications from anticoagulant poisoning. The effect seen in cases of acute ingestion however depends very much on whether warfarin or superwarfarin rodenticides have been ingested. A one-time ingestion of warfarin usually does not lead to any of bleeding problems. (Otherwise, ingestion warfarin rodenticides have to be done repeatedly over a period of days before bleeding can occur). This applies to both rodents and humans. Warfarin poisoning can cause spontaneous bleeding usually from the nose, gums as well as the gastrointestinal and urinary tracts. Haemorrhage into the skin and brain can also occur, though this is less common. Since warfarin takes in 24-60 hours to exert the full anticoagulant effect, most patients will remain asymptomatic long before haemorrhage is likely. Massive anticoagulation ingestions have resulted in ecchymosis, subconjunctival haemorrhage, bleeding gums, or signs of internal haemorrhage (eg. haematemesis, melena, or hematuria). Massive gastrointestinal bleeding and intracranial haemorrhage are considered the most immediate life-threatening complications.
Ingestion of the superwarfarins on the other hand, can produce a prolonged coagulopathy in humans even with a single ingestion. This is thought to be associated with the firmer binding capability of the superwarfarins to the lipophilic sites of the liver. This has been shown with difenacoum, brodifacoum, and chlorphacinone. Six poisoning cases involving adults ingesting 3 to 36 bait packets containing 50g bait each have been reported to cause clinical bleeding and prolonged prothrombin time. The amount ingested did not seem to affect the duration of anticoagulation though the bigger the amount ingested the more severe the clinical and laboratory bleeding occurred. In all these cases, a total of between 42 days to 8 months of blood transfusions and oral vitamin K therapy were required.
Who is at risk for developing complications from anticoagulant poisoning?
Patients at risk to anticoagulant poisoning varies considerably. They may be influenced by a number of factors like medical problems that increase the risk for bleeding, as well as the co-administration with drugs that might interact to potentiate the effects of warfarin. Patient who has a medical history of bleeding tendencies like in peptic ulcer disease and hemophilia, and on medications like aspirin or other drugs that has the ability to displace warfarin from its binding sites are particularly at risk. Such patients need to be monitored closely.
How do you confirm and what is the prognosis?
Diagnosis of poisoning cases involving anticoagulant rodenticides is often based on the history of ingestion and evidence of anticoagulant effect. A history of ingestion that is accompanied with unexplained vitamin K deficiency should point us to anticoagulant exposure. Clinical findings from such exposure is often delayed and as such, decision based on this information is often difficult to do. In this case, the history is very crucial. When in doubt, baseline complete blood counts and prothrombin time should be determined.
How do you manage rodenticide poisoning?
Gastric Decontamination: In the case of a one-time ingestion of warfarin rodenticide, gastric decontamination, either by emesis or gastric lavage are not recommended. Under such circumstances, it is generally felt that such action might cause more harm than good to the patient. Other than this, administration of activated charcoal, with or without a saline cathartic, may be carried out though this is not routinely recommended.
In the case of confirmed or suspected ingestion of the LAAs, gastric lavage should be considered unless it is contraindicated. This may be followed by the administration of a slurry of activated charcoal plus a saline cathartic.
Vitamin K: Specific treatment of superwarfarin-induced coagulopathy includes intravenous or subcutaneous administration of vitamin K1 (phytonadione) and fresh frozen plasma. This approach seems to be the most efficacious form of treatment. Intravenous or intramuscular vitamin K can be started initially, followed by a 40mg dose a day orally for up to 60 days. It should be highlighted that regular oral doses of vitamin K1 are ineffective in counteracting the effect of superwarfarins.
Another form of vitamin K, that is, vitamin K3 (menadione, that requires metabolism by the liver to form vitamin K1) has been suggested for the treatment of anticoagulant poisoning. Its use in patients with generalised haemorrhagic disease however has been questioned. Oral doses of vitamin K3 is felt to be ineffective in cases of superwarfarin ingestion.
All patients should be monitored closely. The prothrombin time should be measured at 24 hours and one week after the last dose of vitamin K before treatment is withdrawn.
OTHER FORMS OF RODENTICIDES
Based on LD50, other rodenticides can be divided as highly, moderately and low toxicity rodenticides.
Highly toxic rodenticides
(single dose LD50 of less 50mg/kg body weight)
Thallium that comes in the form of an odourless, tasteless compound is used to control pests by commercial exterminator. Poisoning can occur from ingestion or from absorption through intact skin. At present, there is no known effective antidote for thallium.
Sodium monofluoroacetate (SMFA or compound 1080), a white, odourless, tasteless, water-soluble salt that looks like flour or baking soda, acts by interfering with the Kreb's cycle. Toxicity occurs upon ingestion, inhalation or absorption through broken skin. Toxic effects expected in humans (extrapolated from rhesus monkeys) includes nausea and apprehension followed by cardiac arrhythmias, seizures and coma. Death may occur as a result of ventricular tachycardia and fibrillation or respiratory failure secondary to pulmonary edema or bronchopneumonia. Currently, there is no antidote for SMFA. Gastric evacuation followed by activated charcoal and cathartic are usually recommended.
Strychnine, a central nervous system stimulant, has been in use as a rodenticide since the 16th century in Germany. Its use has been associated with painful recurrent motor seizures in "decorticate" posturing and trismus, or facial grimacing and medullary paralysis that could lead to death. In between seizures, the patient may remain awake with his muscles in the relaxed state. Management of poisoning cases involving strychnine involves gastric lavage and administration of activated charcoal unless the patient has already presented with symptoms. This is to avoid precipitating the seizures. In fact, it is pertinent in this instance to keep the patient free from any external stimulus.
Zinc phosphide that possesses an unpleasant smell of "rotten fish" is very much liked by rats. As rodenticide, it is usually mixed with a tartar emetic. Upon contact with water it releases a highly toxic gas, phosphine. Patients who are poisoned would develop hypotension, dyspnea, pulmonary edema, circulatory collapse, vomiting, cardiac arrhythmias, convulsions and coma, renal damage, leukopenia, and death in 4 days to 2 weeks. Treatment includes dilution with milk or water, gastric lavage, emesis, and administration of an ionic cathartic.
The elemental yellow phosphorus used as a rodenticide is highly poisonous when compared to the relatively nontoxic red phosphorus. Yellow phosphorus causes skin burns and ingestion of 50mg may be fatal. It is most toxic to the gastrointestinal (GI) tract and liver. Ingestion is usually followed by vomiting which is said to be luminescent and have garlicky odour. Delirium, coma, and death from cardiovascular collapse may ensue. Treatment is essentially the same as for phosphide. Elemental (yellow) phosphorus is rarely used as a rodenticide today.
Arsenic, a white, crystalline powder when ingested, has been associated with problems of dysphagia, muscle cramps, convulsions, vomiting, and bloody diarrhoea. When death occur, it is usually as a result of cardiovascular collapse. The general management of these cases cover aspects of emesis or gastric lavage followed by activated charcoal and an ionic cathartic as well as the use of chelating agents like dimercaprol or penicillamine once absorption has occurred.
Moderately Toxic Rodenticides
(LD50 between 50 and 500 mg body weight)
Alpha-naphthyl-thiourea (ANTU), a derivative of phenyl thiourea but without the bitter taste characteristic of the thiourea, is a relatively selective rodenticide. When used, it kills rats by inducing pulmonary oedema. In humans, ingestion of a large enough quantity may result in similar situation. Patients may present with dyspnea, rales and cyanosis which is the result of pulmonary edema or pleural effusions, and hypothermia. The usual recommendation for managing these cases include inducing of emesis or gastric lavage or emesis followed by administration of a non-oil-based ionic cathartic.
DDT. Poisoning from exposure to DDT can result in symptoms like vomiting, tremors, and convulsions. How much exposure is required to cause severe illness or even death is however not certain. Management from DDT exposure would include emesis or gastric lavage followed by an ionic cathartic.
Low Toxicity Rodenticides
(LD50 between 500 and 5000 mg/kg)
Besides the anticoagulant rodenticides that is classified under this category, there is also the red squill (Urginea maritima) and norbomide. Ingestion of red squill has been associated with problems of cardiac arrythmias particularly in the form of ventricular irritability such as premature ventricular contractions and ventricular fibrillation. Gastrointestinal disturbance is also common, and patient usually presents with abdominal pain, nausea and vomiting. The cardiotoxicity is perhaps best treated with lidocaine or phenytoin. Norbormide has no known human toxicity. In cases of poisoning, emesis and catharsis may be considered if they are easily performed in a particular patient.

Report on Continuing Education for Clinical Toxicology
Dr Abu Bakar Abdul Majeed
Congratulations to all the participants of the 1995 Continuing Education for Clinical Toxicology (CECT) programme. You should all have received the certificate of participation which we have sent to you to be cherished. Consider it as a collectors' item since you are one of the sixty pioneering participants of this programme.
We take our hats off to this group of sixty very responsible and proactive professionals and allied health workers who have taken up the challenge to be involved in Malaysia's first ever structured programme of continuing education.
Among the sixty, there were three medical practitioners and fifty-five pharmacists. There was also a medical assistant and an assistant nurse.
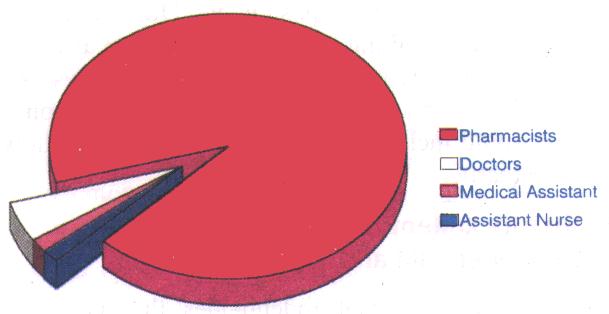
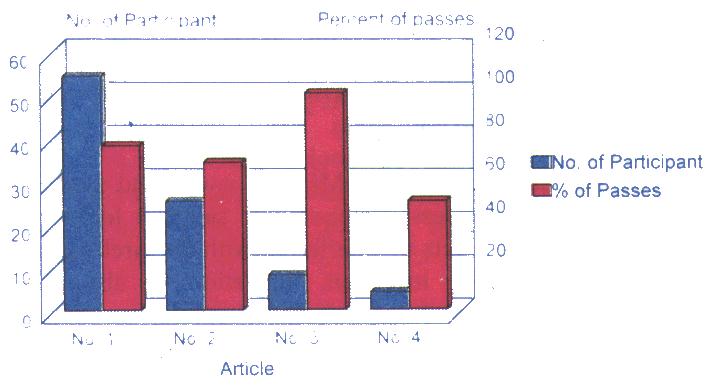
This pioneer group performed remarkably well, thus setting a very high standard for future participants to emulate. Overall performance indicated that there was a more than 75% passing rate (69 passes from 91 attempts).
The CECT programme is conducted on a regular basis through PRN8099, the Professional Bulletin of the National Poison Centre, Malaysia. Participants who satisfactorily complete and submit the CECT questions are awarded one hour of Continuing- Education Credit (1 CEC) unit. To qualify for a unit, a minimum passing grade of 80 percent is required. In 1995, we offered a total of four units of CEC, one each in PRN8099, issue numbers 2,3,4, and 5 (March, May, July and September, respectively).
| Title of CECT article(PRN Consult) | Article appeared in issue no. | CECT questions appeared in issue no. |
|---|---|---|
| Review of Paracetamol Poisoning | 1 | 2 |
| Review of Organophosphate and Carbamate Poisonings | 2 | 3 |
| Review of Salicylate Poisoning | 3 | 4 |
| Review of Cyanide Poisoning | 4 | 5 |
CECT articles and questions of 1995
Bar chart below shows the breakdown of the number of participants according to the four CECT articles and the percentage of passes for each article.
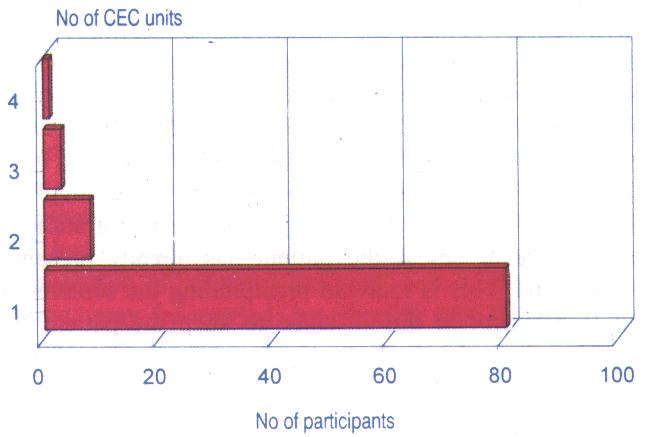
As for the overall number of units accrued by the participants in 1995, the following table aptly provides the breakdown.
In general, we believe that in its inaugural year, the NPC CECT programme has been a success. We are therefore encouraged by this support and we hope to continue with the programme. Beginning 1996, we are changing the format a little bit. The CECT questions now come together with the article. The new format has already been adopted for PRN8099 February 1996 issue (No. 6). You can get a copy of the issue by contacting us. CECT service is now also available on-line via the INTERNET, through http://prn.usm.my. For the time being you can also send your answer through our e-mail:
We strongly urge more health-based professionals and workers to come forward and participate in this exciting continuing education programme. We also welcome suggestions and inputs from our readers. Please do not hesitate to let us know what your think of the programme. Remember, it is never too late to participate. Cheers!.
Poisoning Emergency/ Information
-
Mon-Fri8am-10pm
-
Sat, Sun & Public Holiday8am - 5pm
-
Telephone04 6536 999
-
Telegram chat

